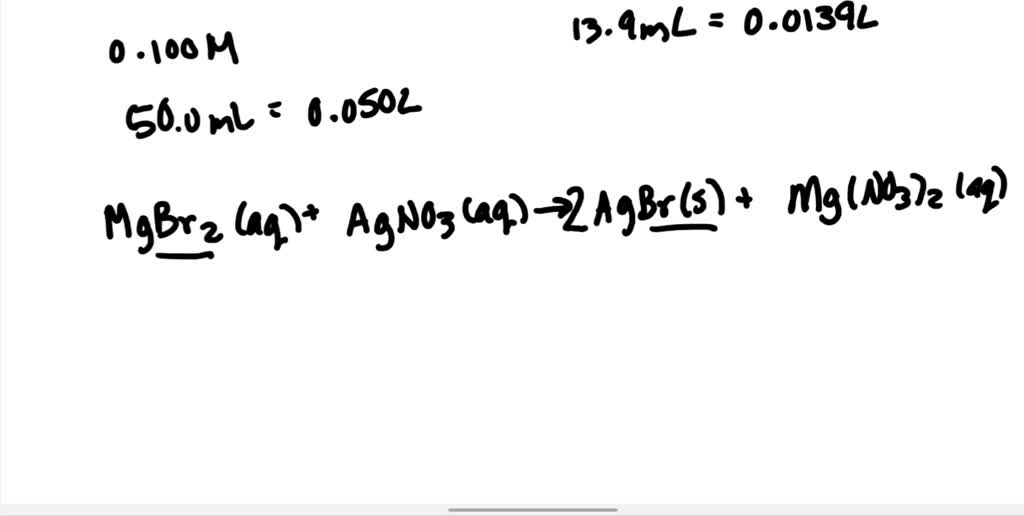Magnesium Bromide Is An Ionic Compound Chemical Formula . When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). formula for ionic compounds. Predict which forms an anion, which forms a cation, and the. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. magnesium and nitrogen react to form an ionic compound. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. It is an essential bromide salt of magnesium. There’s a quick way to determine. Ionic compounds do not exist as molecules. so the formula of the compound that results from reacting magnesium with bromine is: recognize polyatomic ions in chemical formulas.
from www.numerade.com
When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. so the formula of the compound that results from reacting magnesium with bromine is: Ionic compounds do not exist as molecules. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. formula for ionic compounds. recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). magnesium bromide is a white crystalline solid with the chemical formula mgbr2. It is an essential bromide salt of magnesium. There’s a quick way to determine.
SOLVEDGiven that 50.0 mL of 0.100 M magnesium bromide reacts
Magnesium Bromide Is An Ionic Compound Chemical Formula when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. There’s a quick way to determine. recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. magnesium and nitrogen react to form an ionic compound. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. formula for ionic compounds. Predict which forms an anion, which forms a cation, and the. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. so the formula of the compound that results from reacting magnesium with bromine is: It is an essential bromide salt of magnesium. Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\).
From www.slideserve.com
PPT Chapter 3 Molecules, Compounds, and Chemical Equations PowerPoint Magnesium Bromide Is An Ionic Compound Chemical Formula recognize polyatomic ions in chemical formulas. Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. magnesium bromide is a white crystalline ionic compound with. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From hxerslpmd.blob.core.windows.net
Magnesium Bromide Is What Kind Of Compound at Leticia Yazzie blog Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. magnesium and nitrogen react to form an ionic compound. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. formula for ionic compounds. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. . Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.fishersci.com
Fisher Science Education Magnesium Bromide Hexahydrate Fisher Scientific Magnesium Bromide Is An Ionic Compound Chemical Formula When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. so the formula of the compound that results from reacting magnesium with bromine is: Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. It is an essential bromide. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Bromide Is An Ionic Compound Chemical Formula recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. There’s a quick way to determine. Predict which forms an anion, which forms a cation, and the. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. Ionic compounds do. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.youtube.com
How to Balance Mg + Br2 = MgBr2 (Magnesium + Bromine gas) YouTube Magnesium Bromide Is An Ionic Compound Chemical Formula formula for ionic compounds. recognize polyatomic ions in chemical formulas. so the formula of the compound that results from reacting magnesium with bromine is: Ionic compounds do not exist as molecules. There’s a quick way to determine. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Predict which forms an anion, which. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.numerade.com
SOLVED Write the balanced COMPLETE ionic equation for the reaction Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium and nitrogen react to form an ionic compound. Ionic compounds do not exist as molecules. so the formula of the compound that results from reacting magnesium with bromine is: It is an essential bromide salt of magnesium. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). when an ionic compound is. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Magnesium Bromide Is An Ionic Compound Chemical Formula Ionic compounds do not exist as molecules. There’s a quick way to determine. so the formula of the compound that results from reacting magnesium with bromine is: Predict which forms an anion, which forms a cation, and the. It is an essential bromide salt of magnesium. When writing the formula of an ionic compound we use the lowest whole. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. recognize polyatomic ions in chemical formulas. There’s a quick way to determine. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. Ionic compounds do. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From byjus.com
Methyl cyanide on treatment with magnesium bromide followed by Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium and nitrogen react to form an ionic compound. It is an essential bromide salt of magnesium. There’s a quick way to determine. so the formula of the compound that results from reacting magnesium with bromine is: recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\).. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Magnesium Bromide Is An Ionic Compound Chemical Formula It is an essential bromide salt of magnesium. Ionic compounds do not exist as molecules. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. There’s a quick way to determine. formula for ionic compounds. recognize polyatomic ions in chemical formulas. so the formula. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.coursehero.com
[Solved] Cyclohexyl magnesium bromide is reaction with Carbon dioxide Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. There’s a quick way to determine. It is an essential bromide salt of magnesium. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). magnesium and nitrogen react to form an ionic compound. recognize polyatomic ions in chemical formulas. Ionic compounds do not. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.fishersci.com
Magnesium bromide, 98, pure, anhydrous, ACROS Organics Fisher Scientific Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium and nitrogen react to form an ionic compound. Ionic compounds do not exist as molecules. formula for ionic compounds. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. Predict which forms an. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID4566566 Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. magnesium and nitrogen react to form an ionic compound. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. recognize polyatomic ions in chemical formulas. Ionic compounds do not exist as molecules. It is an essential bromide salt of magnesium. so the formula. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.numerade.com
SOLVEDGiven that 50.0 mL of 0.100 M magnesium bromide reacts Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. so the formula of the compound that results from reacting magnesium with bromine is: recognize polyatomic ions in chemical formulas. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). formula for ionic compounds. When writing the formula of an. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From chemistry291.blogspot.com
What Is the Magnesium Bromide Formula? Magnesium Bromide Is An Ionic Compound Chemical Formula Ionic compounds do not exist as molecules. formula for ionic compounds. It is an essential bromide salt of magnesium. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. magnesium and nitrogen react to form an ionic compound. magnesium bromide is a white crystalline. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From dxovlkgkm.blob.core.windows.net
Bromine Potassium Ionic Compound at Richard Walker blog Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. magnesium and nitrogen react to form an ionic compound. recognize polyatomic ions in chemical formulas. formula for ionic compounds. It is an essential bromide salt of magnesium. Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline solid with the chemical formula. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From studylib.net
Ionic Compounds Naming Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. magnesium and nitrogen react to form an ionic compound. so the formula of the compound that results from reacting magnesium with bromine is: formula for ionic compounds. Predict which forms an anion, which forms a cation, and the. magnesium bromide is a white. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.youtube.com
Draw the Lewis Structure of MgBr2 (magnesium bromide) YouTube Magnesium Bromide Is An Ionic Compound Chemical Formula There’s a quick way to determine. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. magnesium and nitrogen react to form an ionic compound. formula for ionic compounds. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Bromide Is An Ionic Compound Chemical Formula recognize polyatomic ions in chemical formulas. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Ionic compounds do not exist as molecules. so the formula of the compound that results from reacting magnesium. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.youtube.com
How to Draw the Lewis Dot Structure for MgBr2 Magnesium bromide YouTube Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. recognize polyatomic ions in chemical formulas. formula for ionic compounds. magnesium and nitrogen react to form an ionic compound. It is an essential bromide salt of magnesium. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions). Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.scibond.com
How to represent a chemical reaction? SciBond Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. recognize polyatomic ions in chemical formulas. It is an essential bromide salt of magnesium. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. so the formula of the compound that results from reacting. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium and nitrogen react to form an ionic compound. There’s a quick way to determine. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. It is an essential bromide salt of magnesium. magnesium bromide is a white crystalline solid with the chemical formula mgbr2.. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From dxoygaprp.blob.core.windows.net
What Is Molecular And Ionic at Patricia Ledbetter blog Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. Ionic compounds do not exist as molecules. magnesium and nitrogen react to form an ionic compound. recognize polyatomic ions in chemical formulas. It is an essential bromide. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From gioasjmdt.blob.core.windows.net
Magnesium Bromide Nomenclature at Carol Richardson blog Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. recognize polyatomic ions in chemical formulas. It is an essential bromide salt of magnesium. There’s a quick way to determine. Predict which forms an anion, which. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.numerade.com
SOLVED Magnesium bromide is a binary ionic compound. From its formula Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. formula for ionic compounds. Ionic compounds do not exist as molecules. magnesium and nitrogen react to form an ionic compound. There’s a quick way to determine. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From athensmutualaid.net
Forming And Naming Binary Ionic Compounds Worksheet Answer Key › Athens Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. recognize polyatomic ions in chemical formulas. There’s a quick way to determine. magnesium bromide is a white crystalline solid with the. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.numerade.com
The electrolysis of an aqueous solution of magnesium bromide (MgBr2 Magnesium Bromide Is An Ionic Compound Chemical Formula There’s a quick way to determine. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. magnesium and nitrogen react to form an ionic compound. so the formula of the compound that results from reacting magnesium with bromine is: When writing the formula of an. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From hxehgfslt.blob.core.windows.net
Does Bromine And Sulfur Form An Ionic Compound at Charlie Wright blog Magnesium Bromide Is An Ionic Compound Chemical Formula Predict which forms an anion, which forms a cation, and the. so the formula of the compound that results from reacting magnesium with bromine is: Ionic compounds do not exist as molecules. It is an essential bromide salt of magnesium. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions). Magnesium Bromide Is An Ionic Compound Chemical Formula.
From studylib.net
Naming Ionic Compounds Answer Key Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. so the formula of the compound that results from reacting magnesium with bromine is: Predict which forms an anion, which forms a cation, and the. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.sarthaks.com
Indicate the expected structure of the organic product when ethyl Magnesium Bromide Is An Ionic Compound Chemical Formula recognize polyatomic ions in chemical formulas. It is an essential bromide salt of magnesium. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. There’s a quick way to determine. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. Ionic compounds do not exist as. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.youtube.com
Is MgBr2 (Magnesium bromide) Ionic or Covalent? YouTube Magnesium Bromide Is An Ionic Compound Chemical Formula formula for ionic compounds. magnesium bromide is a white crystalline solid with the chemical formula mgbr2. There’s a quick way to determine. It is an essential bromide salt of magnesium. magnesium and nitrogen react to form an ionic compound. so the formula of the compound that results from reacting magnesium with bromine is: Ionic compounds do. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.tessshebaylo.com
Net Ionic Equation Ammonium Bromide Dissolved In Water Tessshebaylo Magnesium Bromide Is An Ionic Compound Chemical Formula Ionic compounds do not exist as molecules. formula for ionic compounds. There’s a quick way to determine. Predict which forms an anion, which forms a cation, and the. It is an essential bromide salt of magnesium. recognize polyatomic ions in chemical formulas. When writing the formula of an ionic compound we use the lowest whole number ratio of. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.fishersci.co.uk
Magnesium bromide ethyl etherate, 99, Thermo Scientific Chemicals Magnesium Bromide Is An Ionic Compound Chemical Formula There’s a quick way to determine. so the formula of the compound that results from reacting magnesium with bromine is: magnesium bromide is a white crystalline solid with the chemical formula mgbr2. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. Ionic compounds do. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From terranceaddbenson.blogspot.com
Chemical Formula of Bromide TerranceaddBenson Magnesium Bromide Is An Ionic Compound Chemical Formula It is an essential bromide salt of magnesium. There’s a quick way to determine. Predict which forms an anion, which forms a cation, and the. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ([+] ions) to. Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline ionic. Magnesium Bromide Is An Ionic Compound Chemical Formula.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Bromide Is An Ionic Compound Chemical Formula magnesium bromide is a white crystalline solid with the chemical formula mgbr2. Ionic compounds do not exist as molecules. magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). so the formula of the compound that results from reacting magnesium with bromine is: formula for ionic compounds. when an ionic compound is. Magnesium Bromide Is An Ionic Compound Chemical Formula.