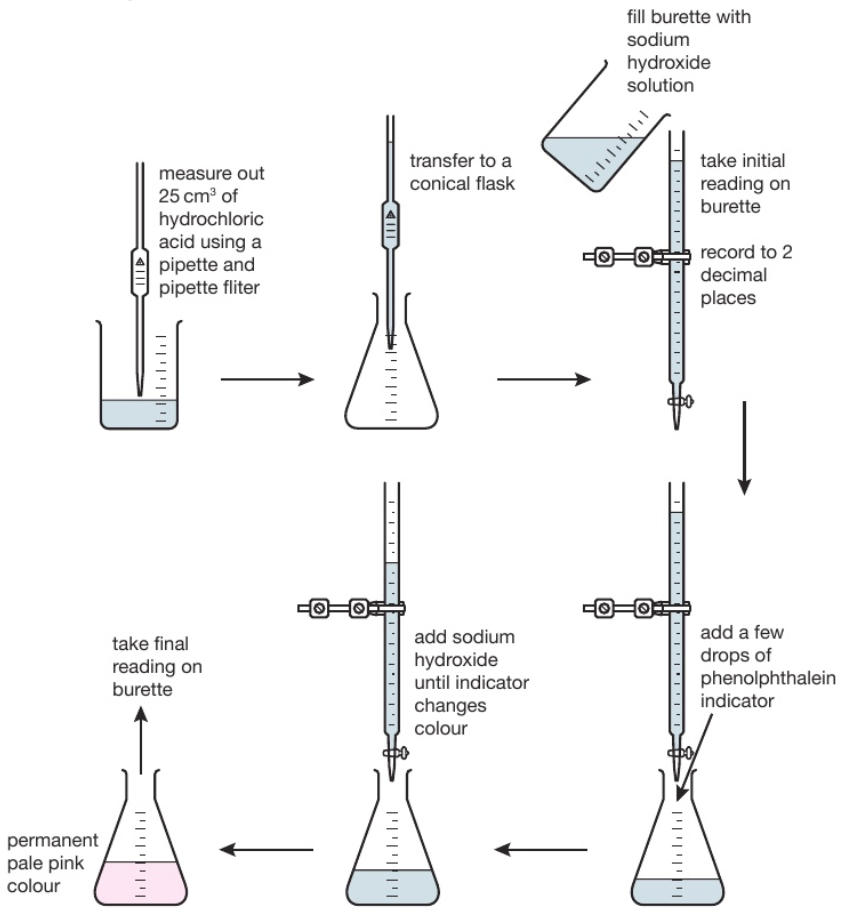
Caustic Titration . use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the chemicals are available in several grades depending on their intended use. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. Solve stoichiometry problems in the. The test methods listed in 1.2 provide.
from www.tutormyself.com
use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. The test methods listed in 1.2 provide. Solve stoichiometry problems in the. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. the chemicals are available in several grades depending on their intended use. Includes kit list and safety instructions. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml.
233 (Triple only) describe how to carry out an acidalkali titration
Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. The test methods listed in 1.2 provide. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the chemicals are available in several grades depending on their intended use. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. Solve stoichiometry problems in the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Includes kit list and safety instructions. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution.
From ar.inspiredpencil.com
Titration Setup Diagram Caustic Titration Includes kit list and safety instructions. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the typical method to. Caustic Titration.
From www.scribd.com
Caustic Soda PDF Chemistry Titration Caustic Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The test methods listed in 1.2 provide. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the shape of a titration curve, a plot of ph versus the amount. Caustic Titration.
From www.nichesolutionsgb.co.uk
Caustic Titration Test Kit Niche Solutions Caustic Titration The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. Solve stoichiometry problems in the. Includes kit list and safety instructions.. Caustic Titration.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Caustic Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. use this class practical to explore titration, producing the salt sodium chloride with sodium. Caustic Titration.
From analysisdoo.com
Titralab AT1000 & KF1000 Analysis Caustic Titration Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The test methods listed in 1.2 provide. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the typical method to determine the strength is to take a titration of a sample. Caustic Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Caustic Titration the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the chemicals are available in several grades depending on their intended use. The. Caustic Titration.
From www.nichesolutionsgb.co.uk
Caustic Titration Test Kit Niche Solutions Caustic Titration the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The test methods listed in 1.2 provide. Sulfuric or hydrochloric acid, using phenolphthalein as an. Caustic Titration.
From dokumen.tips
(PDF) Caustic Soda Titration DOKUMEN.TIPS Caustic Titration the chemicals are available in several grades depending on their intended use. The test methods listed in 1.2 provide. Solve stoichiometry problems in the. Includes kit list and safety instructions. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. At. Caustic Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Caustic Titration the chemicals are available in several grades depending on their intended use. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sulfuric or. Caustic Titration.
From www.maieutapedia.org
Stoichiometry and Titration Maieuta Pedia Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the chemicals are. Caustic Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Caustic Titration The test methods listed in 1.2 provide. Solve stoichiometry problems in the. Includes kit list and safety instructions. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or. Caustic Titration.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Caustic Titration Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The test methods listed in 1.2 provide. the chemicals are available in several grades depending on their intended use. Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the typical method to determine. Caustic Titration.
From www.scribd.com
Determination of Caustic Alkalinity in Water Using Volumetric Titration Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. The strength of sodium hydroxide. Caustic Titration.
From www.youtube.com
Example titration total carbonate caustic alkalinity YouTube Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the typical method to determine the strength is to take. Caustic Titration.
From www.studypool.com
SOLUTION Titration of caustic soda with oxalic acid Studypool Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the chemicals are available in several grades depending on their intended use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. . Caustic Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Caustic Titration Includes kit list and safety instructions. the chemicals are available in several grades depending on their intended use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Solve stoichiometry problems in. Caustic Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the chemicals are available in several grades depending on their intended use. Includes kit list and safety instructions. The test methods listed in 1.2 provide. The strength of sodium hydroxide is. Caustic Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Caustic Titration Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. use this class practical to. Caustic Titration.
From www.studypool.com
SOLUTION Titration of caustic soda with oxalic acid Studypool Caustic Titration the chemicals are available in several grades depending on their intended use. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. . Caustic Titration.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Caustic Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the chemicals are available in several grades depending on their intended use. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. use this class practical to explore titration,. Caustic Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The test methods listed in 1.2 provide. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams. Caustic Titration.
From www.scribd.com
Cyanide and Caustic Soda Titration Procedure Material Safety Data Sheet Caustic Titration Includes kit list and safety instructions. the chemicals are available in several grades depending on their intended use. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. Solve stoichiometry problems in the. The test methods listed in 1.2 provide. Sulfuric. Caustic Titration.
From www.nichesolutionsgb.co.uk
Caustic Titration Test Kit Niche Solutions Caustic Titration The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. The test methods listed in 1.2 provide. use this class. Caustic Titration.
From www.youtube.com
Titration (using phenolphthalein) YouTube Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The test methods listed in 1.2 provide. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. Solve stoichiometry problems in. Caustic Titration.
From acidsandbasesfordummieschem.weebly.com
Titration Acid and Bases for Dummies! Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The test. Caustic Titration.
From www.science-revision.co.uk
Titrations Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The test methods listed in 1.2 provide. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Caustic Titration.
From www.shutterstock.com
Titration Vector Illustration Labeled Educational Chemistry Stock Caustic Titration Solve stoichiometry problems in the. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the chemicals are available in several grades depending. Caustic Titration.
From www.researchgate.net
User interface (UI) for PHREEQNAMDTreat "CausticTitration" modeling Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. the chemicals are available in several grades depending on their intended use.. Caustic Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Caustic Titration the chemicals are available in several grades depending on their intended use. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. Solve stoichiometry problems in the. The test methods listed in 1.2 provide. the shape of a titration curve, a plot of ph versus the amount. Caustic Titration.
From www.nichesolutionsgb.co.uk
Caustic Titration Test Kit Niche Solutions Caustic Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. Solve stoichiometry problems in the.. Caustic Titration.
From www.youtube.com
Sodium Hydroxide Purity Test titration method Caustic Lye titration 🧫🧪🥽 Caustic Titration Includes kit list and safety instructions. the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. the shape of a titration. Caustic Titration.
From sciencing.com
How to Do Titration Calculations Sciencing Caustic Titration The test methods listed in 1.2 provide. Includes kit list and safety instructions. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.. Caustic Titration.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Caustic Titration Includes kit list and safety instructions. Solve stoichiometry problems in the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sulfuric or hydrochloric acid, using phenolphthalein as an indicator. The test methods listed in 1.2 provide. the chemicals are available in several grades depending on their intended use. use. Caustic Titration.
From www.myxxgirl.com
Volumetric Analysis Titration Types Principle Procedure My XXX Hot Girl Caustic Titration the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution. Solve stoichiometry problems in the. The strength of sodium hydroxide is expressed as per cent sodium hydroxide (% naoh) or grams of sodium hydroxide in 100ml. use this class practical to. Caustic Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Caustic Titration the typical method to determine the strength is to take a titration of a sample of the used solution with an acid of known concentration e.g. Solve stoichiometry problems in the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the shape of a titration curve, a plot of. Caustic Titration.