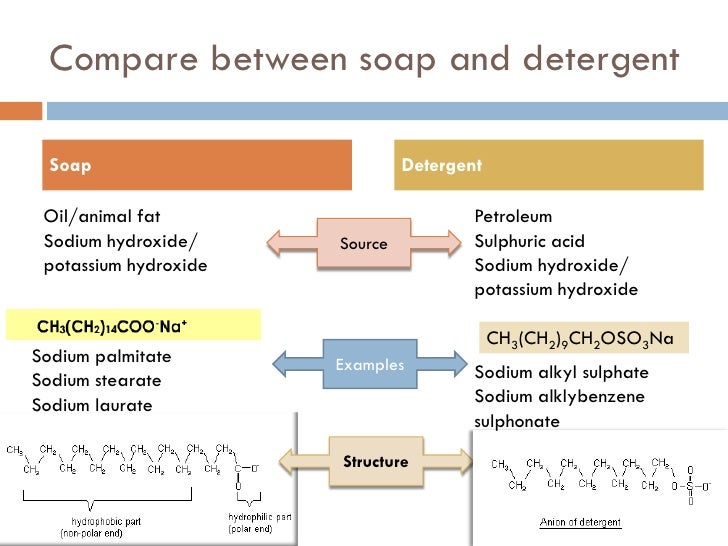
Soaps And Detergents Are Molecular Compounds . The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Surface active molecules present in soaps and detergents dissolve in water. Detergents are synthetic whereas soaps are produced from biomass such. Differences between soaps and detergents. Note that each of these. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Soaps act as cleansers because the two ends of a soap molecule are so different. Most of the dirt is oily in nature and oil does not dissolve in water. Cleansing action of soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. The molecule of soap constitutes sodium or potassium salts of. Hydrophilic heads vary between soaps and the three types of detergents.
from www.slideshare.net
Hydrophilic heads vary between soaps and the three types of detergents. Note that each of these. Soaps act as cleansers because the two ends of a soap molecule are so different. Differences between soaps and detergents. Note that each of these. Most of the dirt is oily in nature and oil does not dissolve in water. The most common examples of such compounds are soaps and detergents, four of which are shown below. Surface active molecules present in soaps and detergents dissolve in water. Cleansing action of soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below.
Soap and Detergents
Soaps And Detergents Are Molecular Compounds Hydrophilic heads vary between soaps and the three types of detergents. Surface active molecules present in soaps and detergents dissolve in water. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Note that each of these. The molecule of soap constitutes sodium or potassium salts of. Differences between soaps and detergents. Soaps act as cleansers because the two ends of a soap molecule are so different. Hydrophilic heads vary between soaps and the three types of detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Cleansing action of soaps and detergents. Detergents are synthetic whereas soaps are produced from biomass such. The most common examples of such compounds are soaps and detergents, four of which are shown below. Most of the dirt is oily in nature and oil does not dissolve in water.
From www.youtube.com
Cleaning agents (Soap and Detergent) Chemistry in every day life NCERT/ NEET/ JEE YouTube Soaps And Detergents Are Molecular Compounds Note that each of these. Differences between soaps and detergents. Surface active molecules present in soaps and detergents dissolve in water. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Cleansing action of soaps and detergents. Detergents are synthetic whereas soaps are produced from. Soaps And Detergents Are Molecular Compounds.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soaps And Detergents Are Molecular Compounds The molecule of soap constitutes sodium or potassium salts of. Hydrophilic heads vary between soaps and the three types of detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. Differences between soaps and detergents. Most of the dirt is oily in nature and oil does not dissolve in water. Detergents are. Soaps And Detergents Are Molecular Compounds.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK. Structural chemical formula Soaps And Detergents Are Molecular Compounds Differences between soaps and detergents. Hydrophilic heads vary between soaps and the three types of detergents. Detergents are synthetic whereas soaps are produced from biomass such. The molecule of soap constitutes sodium or potassium salts of. Cleansing action of soaps and detergents. Note that each of these. Most of the dirt is oily in nature and oil does not dissolve. Soaps And Detergents Are Molecular Compounds.
From thesoapmoleculeco.com
The Soap Molecule Co. Soaps And Detergents Are Molecular Compounds Differences between soaps and detergents. Hydrophilic heads vary between soaps and the three types of detergents. Note that each of these. Surface active molecules present in soaps and detergents dissolve in water. Cleansing action of soaps and detergents. Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such. Most of the dirt is oily in. Soaps And Detergents Are Molecular Compounds.
From www.researchgate.net
Chemical structures of some commonly used detergents SDS, sodium... Download Scientific Diagram Soaps And Detergents Are Molecular Compounds Hydrophilic heads vary between soaps and the three types of detergents. Cleansing action of soaps and detergents. Differences between soaps and detergents. Surface active molecules present in soaps and detergents dissolve in water. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Detergents are synthetic whereas soaps. Soaps And Detergents Are Molecular Compounds.
From www.coursehero.com
[Solved] Draw the chemical structure of a soap and a detergent (choose only... Course Hero Soaps And Detergents Are Molecular Compounds Detergents are synthetic whereas soaps are produced from biomass such. The molecule of soap constitutes sodium or potassium salts of. Note that each of these. The most common examples of such compounds are soaps and detergents, four of which are shown below. Hydrophilic heads vary between soaps and the three types of detergents. The most common examples of such compounds. Soaps And Detergents Are Molecular Compounds.
From www.defeatdd.org
How does soap actually work? Soaps And Detergents Are Molecular Compounds Most of the dirt is oily in nature and oil does not dissolve in water. Detergents are synthetic whereas soaps are produced from biomass such. Hydrophilic heads vary between soaps and the three types of detergents. Note that each of these. The molecule of soap constitutes sodium or potassium salts of. Cleansing action of soaps and detergents. The most common. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Carbon and its compounds Soaps and detergents class 10 science NCERT YouTube Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are synthetic whereas soaps are produced from biomass such. Note that each of these. Differences between soaps and detergents. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in. Soaps And Detergents Are Molecular Compounds.
From ar.inspiredpencil.com
Soap Molecule Structure Soaps And Detergents Are Molecular Compounds The molecule of soap constitutes sodium or potassium salts of. Cleansing action of soaps and detergents. Hydrophilic heads vary between soaps and the three types of detergents. Surface active molecules present in soaps and detergents dissolve in water. Note that each of these. Most of the dirt is oily in nature and oil does not dissolve in water. The most. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Carbon and its compounds class 10 structure of soap molecule class 10 YouTube Soaps And Detergents Are Molecular Compounds Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Detergents are synthetic whereas soaps are produced from biomass such. The most common examples of such compounds are soaps and detergents, four of which are shown below. Most of the dirt is oily in nature. Soaps And Detergents Are Molecular Compounds.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID6534085 Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. Cleansing action of soaps and detergents. Soaps act as cleansers because the two ends of a soap molecule are so different. Differences between soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below.. Soaps And Detergents Are Molecular Compounds.
From spmchemistry.blog.onlinetuition.com.my
Detergent SPM Chemistry Soaps And Detergents Are Molecular Compounds Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Cleansing action of soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents // HSC Chemistry YouTube Soaps And Detergents Are Molecular Compounds Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes sodium or potassium salts of. Cleansing action of soaps and detergents. Detergents are synthetic whereas soaps are produced from biomass such. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that. Soaps And Detergents Are Molecular Compounds.
From www.slideserve.com
PPT Soap and Detergents Manufacture PowerPoint Presentation ID2067968 Soaps And Detergents Are Molecular Compounds Most of the dirt is oily in nature and oil does not dissolve in water. Differences between soaps and detergents. Soaps act as cleansers because the two ends of a soap molecule are so different. Note that each of these. Hydrophilic heads vary between soaps and the three types of detergents. The molecule of soap constitutes sodium or potassium salts. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Class 10 Science Comparison between Soaps & Detergents Carbon and its Compounds YouTube Soaps And Detergents Are Molecular Compounds Soaps act as cleansers because the two ends of a soap molecule are so different. The molecule of soap constitutes sodium or potassium salts of. Note that each of these. Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as. Soaps And Detergents Are Molecular Compounds.
From homedecorjunky.blogspot.com
Soap Organic Compound Sodium Stearate Molecule Sodium Stearate Molecular Model Organic Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. Surface active molecules present in soaps and detergents dissolve in water. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Cleansing action of soaps and detergents. Differences between soaps and. Soaps And Detergents Are Molecular Compounds.
From study.com
Soap & Detergent Chemistry, Types & Differences Lesson Soaps And Detergents Are Molecular Compounds Differences between soaps and detergents. Surface active molecules present in soaps and detergents dissolve in water. Detergents are synthetic whereas soaps are produced from biomass such. Soaps act as cleansers because the two ends of a soap molecule are so different. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that. Soaps And Detergents Are Molecular Compounds.
From aliceinchemiland.blogspot.com
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Detergent Soaps And Detergents Are Molecular Compounds Cleansing action of soaps and detergents. Hydrophilic heads vary between soaps and the three types of detergents. Most of the dirt is oily in nature and oil does not dissolve in water. The most common examples of such compounds are soaps and detergents, four of which are shown below. Surface active molecules present in soaps and detergents dissolve in water.. Soaps And Detergents Are Molecular Compounds.
From cosmosmagazine.com
The chemistry of soap Soaps And Detergents Are Molecular Compounds Most of the dirt is oily in nature and oil does not dissolve in water. Soaps act as cleansers because the two ends of a soap molecule are so different. Differences between soaps and detergents. Hydrophilic heads vary between soaps and the three types of detergents. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century. Soaps And Detergents Are Molecular Compounds.
From www.slideserve.com
PPT Chapter 30 Detergents PowerPoint Presentation, free download ID3099938 Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. Most of the dirt is oily in nature and oil does not dissolve in water. The most common examples of such compounds are soaps and detergents, four of which are shown below. The molecule of soap constitutes sodium or potassium salts of. Note. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Soaps and detergents Carbon and its compounds Class 10 Chemistry Khan Academy YouTube Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. The most common examples of such compounds are soaps and detergents, four of which are shown below. Soaps act as cleansers because the two ends of a soap molecule are so different. Most of the dirt is oily in nature and oil does. Soaps And Detergents Are Molecular Compounds.
From brainly.in
what is the difference between the molecules of soap and detergents chemically explain the Soaps And Detergents Are Molecular Compounds Cleansing action of soaps and detergents. Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such. Surface active molecules present in soaps and detergents dissolve in water. Note that each of these. The molecule of soap constitutes sodium or potassium salts of. Detergents, often referred to as synthetic detergents, were introduced in the early 20th. Soaps And Detergents Are Molecular Compounds.
From studylib.net
THE SCIENCE OF SOAPS AND DETERGENTS Soaps And Detergents Are Molecular Compounds The molecule of soap constitutes sodium or potassium salts of. Surface active molecules present in soaps and detergents dissolve in water. Most of the dirt is oily in nature and oil does not dissolve in water. Hydrophilic heads vary between soaps and the three types of detergents. The most common examples of such compounds are soaps and detergents, four of. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Soaps and Detergents ( Carbon and its Compounds ) Class 10 YouTube Soaps And Detergents Are Molecular Compounds Detergents are synthetic whereas soaps are produced from biomass such. Differences between soaps and detergents. Soaps act as cleansers because the two ends of a soap molecule are so different. Cleansing action of soaps and detergents. Most of the dirt is oily in nature and oil does not dissolve in water. The most common examples of such compounds are soaps. Soaps And Detergents Are Molecular Compounds.
From www.slideshare.net
Chemistry of soaps Soaps And Detergents Are Molecular Compounds The most common examples of such compounds are soaps and detergents, four of which are shown below. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Cleansing action of soaps and detergents. Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such.. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Soaps & DetergentClass 10 Science CBSE(NCERT)Ch4Carbon &it's compounds YouTube Soaps And Detergents Are Molecular Compounds Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Note that each of these. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Detergents are synthetic whereas soaps are produced from. Soaps And Detergents Are Molecular Compounds.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Soaps And Detergents Are Molecular Compounds Note that each of these. Most of the dirt is oily in nature and oil does not dissolve in water. Note that each of these. Hydrophilic heads vary between soaps and the three types of detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. Surface active molecules present in soaps and. Soaps And Detergents Are Molecular Compounds.
From slideplayer.com
Higher Chemistry Detergents Clean Chemistry ppt download Soaps And Detergents Are Molecular Compounds Soaps act as cleansers because the two ends of a soap molecule are so different. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to the limitations of soap in hard water. Surface active molecules present in soaps and detergents dissolve in water. Most of the dirt is oily in nature and. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Soaps and Detergents/Carbon and its Compounds Structure of Micelle YouTube Soaps And Detergents Are Molecular Compounds The molecule of soap constitutes sodium or potassium salts of. Most of the dirt is oily in nature and oil does not dissolve in water. Detergents are synthetic whereas soaps are produced from biomass such. Hydrophilic heads vary between soaps and the three types of detergents. Note that each of these. Differences between soaps and detergents. Cleansing action of soaps. Soaps And Detergents Are Molecular Compounds.
From easychem.com.au
The Difference Between Soaps And Synthetic Detergents EasyChem Australia Soaps And Detergents Are Molecular Compounds The molecule of soap constitutes sodium or potassium salts of. Detergents are synthetic whereas soaps are produced from biomass such. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these. Detergents, often referred to as synthetic detergents, were introduced in the early 20th century as a response to. Soaps And Detergents Are Molecular Compounds.
From www.slideshare.net
Soap and Detergents Soaps And Detergents Are Molecular Compounds Hydrophilic heads vary between soaps and the three types of detergents. Note that each of these. Note that each of these. Soaps act as cleansers because the two ends of a soap molecule are so different. Differences between soaps and detergents. Most of the dirt is oily in nature and oil does not dissolve in water. Cleansing action of soaps. Soaps And Detergents Are Molecular Compounds.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soaps And Detergents Are Molecular Compounds Soaps act as cleansers because the two ends of a soap molecule are so different. Note that each of these. Detergents are synthetic whereas soaps are produced from biomass such. The most common examples of such compounds are soaps and detergents, four of which are shown below. The molecule of soap constitutes sodium or potassium salts of. Most of the. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Soaps and detergents Carbon and its compounds Class 10 Chemistry Chapter 4 YouTube Soaps And Detergents Are Molecular Compounds Differences between soaps and detergents. Note that each of these. Cleansing action of soaps and detergents. The most common examples of such compounds are soaps and detergents, four of which are shown below. The most common examples of such compounds are soaps and detergents, four of which are shown below. Hydrophilic heads vary between soaps and the three types of. Soaps And Detergents Are Molecular Compounds.
From ar.inspiredpencil.com
Soap Molecule Structure Soaps And Detergents Are Molecular Compounds Most of the dirt is oily in nature and oil does not dissolve in water. Hydrophilic heads vary between soaps and the three types of detergents. Surface active molecules present in soaps and detergents dissolve in water. Detergents are synthetic whereas soaps are produced from biomass such. Differences between soaps and detergents. The most common examples of such compounds are. Soaps And Detergents Are Molecular Compounds.
From www.youtube.com
Class 10th Science Soaps & Detergents Chapter 4 Carbon and its Compounds NCERT YouTube Soaps And Detergents Are Molecular Compounds Surface active molecules present in soaps and detergents dissolve in water. Note that each of these. Cleansing action of soaps and detergents. The molecule of soap constitutes sodium or potassium salts of. The most common examples of such compounds are soaps and detergents, four of which are shown below. Differences between soaps and detergents. Detergents are synthetic whereas soaps are. Soaps And Detergents Are Molecular Compounds.