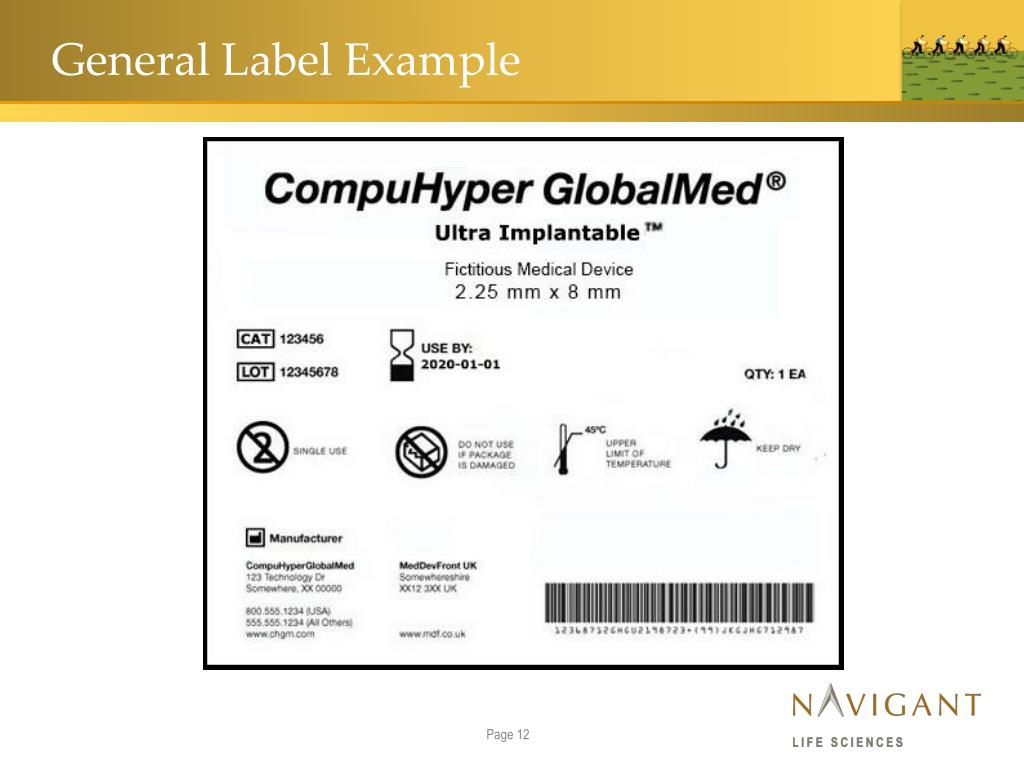
Medical Device Label Validation . Indicates a medical device that contains or incorporates human blood or plasma derivatives. In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. The embedded cross may be deleted or replaced. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that.
from www.slideserve.com
In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. The embedded cross may be deleted or replaced. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that.
PPT Medical Device Labeling PowerPoint Presentation, free download
Medical Device Label Validation The embedded cross may be deleted or replaced. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical devices are products or equipment intended for a medical purpose. Indicates a medical device that contains or incorporates human blood or plasma derivatives. The embedded cross may be deleted or replaced. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. In the european union (eu) they must undergo a conformity assessment to.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Label Validation Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Medical devices are products or equipment. Medical Device Label Validation.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Medical devices are products or equipment intended for a medical purpose. Medical device labelling validation is an essential process. Medical Device Label Validation.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical devices are products or equipment intended for a medical purpose. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. In the european union (eu). Medical Device Label Validation.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Device Label Validation Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Medical devices are products or equipment intended for a medical purpose. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The embedded cross may be deleted or replaced. Labeling and packaging. Medical Device Label Validation.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Label Validation Medical devices are products or equipment intended for a medical purpose. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can. Medical Device Label Validation.
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Medical Device Label Validation Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Indicates a medical device that contains or incorporates human blood or plasma derivatives. The embedded cross may be. Medical Device Label Validation.
From www.flexo-graphics.com
Medical Device Label Printing Medical Custom Labels Medical Device Label Validation The embedded cross may be deleted or replaced. In the european union (eu) they must undergo a conformity assessment to. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Provide guidance to medical device. Medical Device Label Validation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Label Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The embedded cross may be deleted or replaced. Indicates a medical device that contains or incorporates human blood or plasma derivatives. In the european union (eu) they must undergo a conformity assessment to. Labeling and packaging is key to ensure that. Medical Device Label Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Label Validation In the european union (eu) they must undergo a conformity assessment to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical devices are products or equipment intended. Medical Device Label Validation.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Validation of Cleaning Medical Device Label Validation The embedded cross may be deleted or replaced. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical devices are products or equipment intended for a medical purpose. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Provide guidance to medical device manufacturers. Medical Device Label Validation.
From stableshvf.com
Free Medical Device Validation Report Template Word Example Stableshvf Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The embedded cross may be deleted or replaced. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively. Medical Device Label Validation.
From www.camcode.com
UDI Labels (Unique Device Identification) for Medical Devices Camcode Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. The embedded cross may be deleted or replaced. Provide guidance to medical device manufacturers in the complex activities involved. Medical Device Label Validation.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. The embedded cross may be deleted or replaced. In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device. Medical Device Label Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Label Validation Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. In the european union (eu) they must undergo a conformity assessment to. The embedded cross may be deleted or replaced. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Indicates a. Medical Device Label Validation.
From www.youtube.com
Difference between Verification and Validation ISO 9001 Definitions Medical Device Label Validation Medical devices are products or equipment intended for a medical purpose. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device labelling validation is. Medical Device Label Validation.
From www.en-standard.eu
BS ISO 1522322010 Medical devices. Symbols to be used with medical Medical Device Label Validation The embedded cross may be deleted or replaced. Indicates a medical device that contains or incorporates human blood or plasma derivatives. In the european union (eu) they must undergo a conformity assessment to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling and packaging is key to ensure that. Medical Device Label Validation.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Labeling and packaging is key to. Medical Device Label Validation.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In the european union (eu) they. Medical Device Label Validation.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Validation In the european union (eu) they must undergo a conformity assessment to. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The embedded cross may be deleted or replaced. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that. Medical Device Label Validation.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Medical devices are products or equipment intended for a. Medical Device Label Validation.
From www.enlabel.com
Unique Device Identification UDI enLabel Global Services Medical Device Label Validation Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The embedded cross may be deleted or replaced. Labeling and packaging is key to ensure that a medical device or in vitro. Medical Device Label Validation.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. In the european union (eu) they must undergo a conformity assessment to. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of. Medical Device Label Validation.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Medical devices are products or equipment. Medical Device Label Validation.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Medical Device Label Validation Indicates a medical device that contains or incorporates human blood or plasma derivatives. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Medical device labelling validation is an. Medical Device Label Validation.
From stableshvf.com
Free Medical Device Validation Report Template Excel Sample Stableshvf Medical Device Label Validation Medical devices are products or equipment intended for a medical purpose. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The embedded cross may be deleted or replaced. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr).. Medical Device Label Validation.
From www.presentationeze.com
QSIT Medical Device Validation requirements PresentationEZE Medical Device Label Validation Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely.. Medical Device Label Validation.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Label Validation Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical devices are products or equipment intended for a medical purpose. Provide guidance to medical device. Medical Device Label Validation.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Label Validation Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In the european union (eu) they must undergo a conformity assessment to. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. The embedded cross may. Medical Device Label Validation.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Label Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that.. Medical Device Label Validation.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Label Validation Medical device labelling validation is an essential process in the medical device industry, encompassing the verification and assurance that. Medical devices are products or equipment intended for a medical purpose. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can. Medical Device Label Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Label Validation In the european union (eu) they must undergo a conformity assessment to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Indicates a medical device that contains or incorporates human blood or plasma derivatives. Medical devices are products or equipment intended for a medical purpose. Labeling regulations pertaining to medical. Medical Device Label Validation.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Validation Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. Medical device labelling validation is an essential process in the medical device industry, encompassing the. Medical Device Label Validation.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Label Validation In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. The embedded cross may be deleted or replaced. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Indicates a medical device that contains or. Medical Device Label Validation.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Label Validation Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In the european union (eu) they must undergo a conformity assessment to. Provide guidance to medical. Medical Device Label Validation.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Medical Device Label Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical devices are products or equipment intended for a medical purpose. Indicates a medical device that contains or incorporates. Medical Device Label Validation.