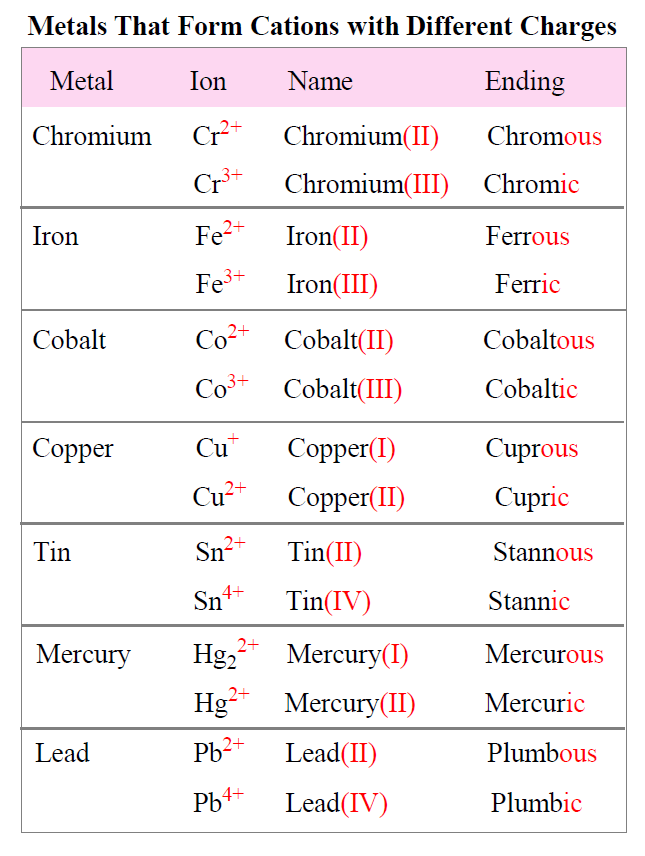
What Is A Cation Chemistry . Because they have opposite electrical charges,. A cation is an ion that has lost one or more electrons, giving a net positive charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are ions with a net positive charge. Groups 1 and 2 elements form cations. Anions are ions with a net negative charge. Cations are named according to the parent. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Because one or more electrons are removed to form a cation, the cation of an atom is. For a cation to form, one or more electrons must be lost, typically pulled away. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Cations are formed by the loss of one or two electrons from an element.
from general.chemistrysteps.com
Conversely, ions that contain more electrons than protons have a net negative charge and are. Groups 1 and 2 elements form cations. Because they have opposite electrical charges,. Cations are formed by the loss of one or two electrons from an element. A cation has more protons than electrons, consequently giving it a net positive charge. Anions are ions with a net negative charge. Because one or more electrons are removed to form a cation, the cation of an atom is. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are ions with a net positive charge. For a cation to form, one or more electrons must be lost, typically pulled away.
Naming Ionic Compounds Chemistry Steps
What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. For a cation to form, one or more electrons must be lost, typically pulled away. Cations are named according to the parent. Because one or more electrons are removed to form a cation, the cation of an atom is. Anions are ions with a net negative charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. Cations are ions with a net positive charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Groups 1 and 2 elements form cations. A cation is an ion that has lost one or more electrons, giving a net positive charge. Because they have opposite electrical charges,. Cations are formed by the loss of one or two electrons from an element. A cation has more protons than electrons, consequently giving it a net positive charge.
From www.aquaportail.com
Cation définition et explications What Is A Cation Chemistry A cation is an ion that has lost one or more electrons, giving a net positive charge. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Because one or more electrons are removed to form a cation, the cation of an atom is. Ions that contain fewer electrons than protons have. What Is A Cation Chemistry.
From www.slideserve.com
PPT CHEMISTRY The Central Science 9th Edition PowerPoint Presentation What Is A Cation Chemistry Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are ions with a net positive charge. A cation is an ion that has lost one or more electrons, giving a net positive charge. Learn about and revise the structure. What Is A Cation Chemistry.
From www.youtube.com
IONS CATION & ANION [ AboodyTV ] Chemistry YouTube What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. Cations are ions with a net positive charge. Groups 1 and 2 elements form cations. Ions that contain fewer electrons than protons have a net positive charge and are called cations. A cation has more protons than electrons, consequently giving it a net positive charge.. What Is A Cation Chemistry.
From mccarrontheyess.blogspot.com
Give Two Examples of Monatomic Cation McCarron Theyess What Is A Cation Chemistry Cations are named according to the parent. A cation has more protons than electrons, consequently giving it a net positive charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation is an ion that has lost one or more electrons, giving a net positive charge. Anions are ions with a net negative. What Is A Cation Chemistry.
From socratic.org
Question 587a9 Socratic What Is A Cation Chemistry Anions are ions with a net negative charge. For a cation to form, one or more electrons must be lost, typically pulled away. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. A cation has more protons than electrons, consequently giving it a net positive charge. Ions that contain fewer electrons. What Is A Cation Chemistry.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Is A Cation Chemistry Cations are named according to the parent. Anions are ions with a net negative charge. Because one or more electrons are removed to form a cation, the cation of an atom is. Conversely, ions that contain more electrons than protons have a net negative charge and are. Cations are formed by the loss of one or two electrons from an. What Is A Cation Chemistry.
From www.youtube.com
what is an Ion? Cation and Anion Chemistry YouTube What Is A Cation Chemistry Because one or more electrons are removed to form a cation, the cation of an atom is. Cations are ions with a net positive charge. Groups 1 and 2 elements form cations. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are named according to the parent. Conversely, ions that. What Is A Cation Chemistry.
From www.sliderbase.com
Chemistry Presentation Chemistry What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are ions with a net positive charge. Because they have opposite electrical charges,. Groups 1 and 2 elements form cations. Conversely, ions that contain more electrons than protons have a. What Is A Cation Chemistry.
From www.animalia-life.club
Cation And Anion What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. A cation has more protons than electrons, consequently giving it a net positive charge. Anions are ions with a net negative charge. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are ions with a. What Is A Cation Chemistry.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences What Is A Cation Chemistry Conversely, ions that contain more electrons than protons have a net negative charge and are. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Because one or more electrons are removed to form a cation, the cation of an atom is. Cations are ions with a net positive charge. Groups 1. What Is A Cation Chemistry.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Is A Cation Chemistry Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Anions are ions with a net negative charge. For a cation to form, one or more electrons must be lost, typically pulled away. Cations are named according to the parent. A cation has more protons than electrons, consequently giving it a net. What Is A Cation Chemistry.
From study.com
Cation Definition & Examples Video & Lesson Transcript What Is A Cation Chemistry Anions are ions with a net negative charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. For a cation to form, one or more electrons must be lost, typically pulled away. Cations are formed by the loss of one or two electrons from an element. Because they have opposite electrical charges,. Groups 1. What Is A Cation Chemistry.
From byjus.com
Systematic Analysis of Cations Chemistry Practicals Class 12 What Is A Cation Chemistry Cations are formed by the loss of one or two electrons from an element. Cations are named according to the parent. Because one or more electrons are removed to form a cation, the cation of an atom is. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Learn about and revise the structure. What Is A Cation Chemistry.
From www.slideserve.com
PPT Binary Compounds PowerPoint Presentation, free download ID2938672 What Is A Cation Chemistry Cations are formed by the loss of one or two electrons from an element. A cation is an ion that has lost one or more electrons, giving a net positive charge. Anions are ions with a net negative charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. Cations are ions with a net. What Is A Cation Chemistry.
From chem.libretexts.org
Chapter 10.3 Naming Ionic Compounds Chemistry LibreTexts What Is A Cation Chemistry Cations are formed by the loss of one or two electrons from an element. Cations are ions with a net positive charge. A cation is an ion that has lost one or more electrons, giving a net positive charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Groups 1 and 2 elements. What Is A Cation Chemistry.
From www.youtube.com
What is an Ion Definition, Formation ,Examples and types of ions What Is A Cation Chemistry Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. For a cation to form, one or more electrons must be lost, typically pulled away. Groups 1 and 2 elements form cations. Cations are formed by the loss of one or two electrons from an element. Conversely, ions that contain more electrons. What Is A Cation Chemistry.
From studymind.co.uk
Identifying Ions (GCSE Chemistry) Study Mind What Is A Cation Chemistry A cation has more protons than electrons, consequently giving it a net positive charge. Groups 1 and 2 elements form cations. Cations are formed by the loss of one or two electrons from an element. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are named according to the parent.. What Is A Cation Chemistry.
From aboveknowledge.blogspot.com
How to remember the charge of cation and anion What Is A Cation Chemistry Groups 1 and 2 elements form cations. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Cations are ions with a net positive charge. Because they have opposite electrical charges,. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are named according to. What Is A Cation Chemistry.
From www.thoughtco.com
The Difference Between a Cation and an Anion What Is A Cation Chemistry Because one or more electrons are removed to form a cation, the cation of an atom is. Anions are ions with a net negative charge. A cation has more protons than electrons, consequently giving it a net positive charge. Because they have opposite electrical charges,. Groups 1 and 2 elements form cations. Ions that contain fewer electrons than protons have. What Is A Cation Chemistry.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. Cations are ions with a net positive charge. A cation has more protons than electrons, consequently giving it a net positive charge. Anions are ions with a net negative charge. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse. What Is A Cation Chemistry.
From www.slideserve.com
PPT Cations PowerPoint Presentation, free download ID3142779 What Is A Cation Chemistry Because one or more electrons are removed to form a cation, the cation of an atom is. Ions that contain fewer electrons than protons have a net positive charge and are called cations. For a cation to form, one or more electrons must be lost, typically pulled away. Learn about and revise the structure of atoms, atoms and isotopes and. What Is A Cation Chemistry.
From in.pinterest.com
two tables showing the different types of common anions What Is A Cation Chemistry Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are named according to the parent. Anions are ions with a net negative charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Because they have opposite electrical charges,. For a cation to form,. What Is A Cation Chemistry.
From www.geeksforgeeks.org
Cations and Anions Difference between Cations and Anions What Is A Cation Chemistry Anions are ions with a net negative charge. Cations are formed by the loss of one or two electrons from an element. A cation is an ion that has lost one or more electrons, giving a net positive charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. Because they have opposite electrical charges,.. What Is A Cation Chemistry.
From adrienj.tinosmarble.com
Cations vs Anions Difference Between Cations and Anions with Examples What Is A Cation Chemistry Ions that contain fewer electrons than protons have a net positive charge and are called cations. A cation is an ion that has lost one or more electrons, giving a net positive charge. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are ions with a net positive charge. Groups 1 and 2 elements. What Is A Cation Chemistry.
From chemistry.about.com
What Is the Difference Between a Cation and an Anion? What Is A Cation Chemistry Cations are formed by the loss of one or two electrons from an element. A cation is an ion that has lost one or more electrons, giving a net positive charge. Anions are ions with a net negative charge. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Cations are named. What Is A Cation Chemistry.
From www.pinterest.com
Cations and anions are positively and negatively charged atoms What Is A Cation Chemistry A cation has more protons than electrons, consequently giving it a net positive charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. For a cation to form, one or more electrons must be lost, typically. What Is A Cation Chemistry.
From jackwestin.com
Anion Cation Common Names Formulas And Charges For Familiar Ions Ions What Is A Cation Chemistry For a cation to form, one or more electrons must be lost, typically pulled away. A cation is an ion that has lost one or more electrons, giving a net positive charge. Cations are named according to the parent. Because one or more electrons are removed to form a cation, the cation of an atom is. Anions are ions with. What Is A Cation Chemistry.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps What Is A Cation Chemistry Cations are ions with a net positive charge. For a cation to form, one or more electrons must be lost, typically pulled away. Cations are formed by the loss of one or two electrons from an element. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are named according to the parent. A cation. What Is A Cation Chemistry.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell What Is A Cation Chemistry Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Anions are ions with a net negative charge. Because one or more electrons are removed to form a cation, the cation of an atom is. A cation is an ion that has lost one or more electrons, giving a net positive charge.. What Is A Cation Chemistry.
From www.slideserve.com
PPT Elements PowerPoint Presentation, free download ID1588776 What Is A Cation Chemistry Cations are formed by the loss of one or two electrons from an element. Because one or more electrons are removed to form a cation, the cation of an atom is. Groups 1 and 2 elements form cations. Because they have opposite electrical charges,. A cation has more protons than electrons, consequently giving it a net positive charge. Conversely, ions. What Is A Cation Chemistry.
From superhumanacademy.com
How to Memorize Polyatomic Ions & Chemical Formulas SuperHuman Academy What Is A Cation Chemistry Because one or more electrons are removed to form a cation, the cation of an atom is. Because they have opposite electrical charges,. Cations are ions with a net positive charge. Cations are named according to the parent. Anions are ions with a net negative charge. Conversely, ions that contain more electrons than protons have a net negative charge and. What Is A Cation Chemistry.
From www.animalia-life.club
Cation And Anion What Is A Cation Chemistry Conversely, ions that contain more electrons than protons have a net negative charge and are. Groups 1 and 2 elements form cations. A cation has more protons than electrons, consequently giving it a net positive charge. A cation is an ion that has lost one or more electrons, giving a net positive charge. For a cation to form, one or. What Is A Cation Chemistry.
From www.teachoo.com
Ions Meaning and Examples [in Chemistry] Teachoo Concepts What Is A Cation Chemistry Anions are ions with a net negative charge. Because they have opposite electrical charges,. A cation has more protons than electrons, consequently giving it a net positive charge. Cations are ions with a net positive charge. Conversely, ions that contain more electrons than protons have a net negative charge and are. For a cation to form, one or more electrons. What Is A Cation Chemistry.
From receivinghelpdesk.com
How Are Cations And Anions Formed From Neutral Atoms What Is A Cation Chemistry Because one or more electrons are removed to form a cation, the cation of an atom is. Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation has more protons than electrons, consequently giving it a net positive charge. Learn about and revise the structure of atoms, atoms and isotopes and ions with. What Is A Cation Chemistry.
From www.dreamstime.com
Cations and Anions. Structure of Ions Stock Vector Illustration of What Is A Cation Chemistry A cation has more protons than electrons, consequently giving it a net positive charge. Anions are ions with a net negative charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Because one or more electrons are removed to form a cation, the cation of an atom is. Cations are formed by the. What Is A Cation Chemistry.