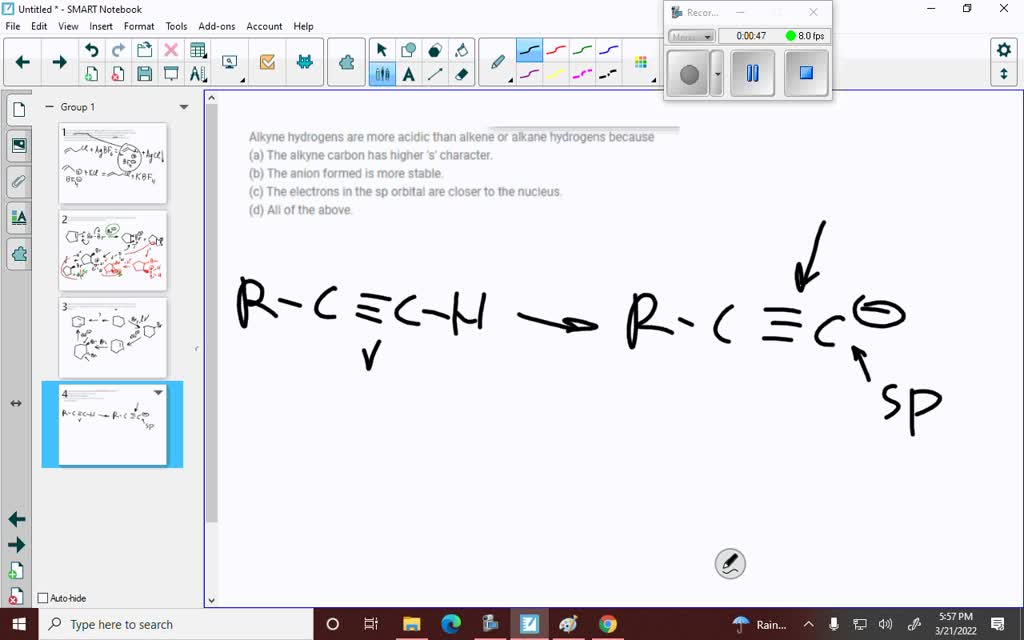
Which Is More Acidic Alkane Alkene Or Alkyne . When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Why are alkynes acidic in comparison to alkanes and alkenes? Assertion :alkenes and alkynes are more reactive than alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the.
from www.numerade.com
Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Assertion :alkenes and alkynes are more reactive than alkanes. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. Why are alkynes acidic in comparison to alkanes and alkenes? The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a.
SOLVED Alkyne hydrogens are more acidic than alkene or alkane
Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Assertion :alkenes and alkynes are more reactive than alkanes. Why are alkynes acidic in comparison to alkanes and alkenes?
From slideplayer.com
Organic Chemistry CHEM ppt download Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes >. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.youtube.com
Differences Between Alkane, Alkene, and Alkyne. YouTube Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how. Which Is More Acidic Alkane Alkene Or Alkyne.
From narodnatribuna.info
14 Alkene Alkyne Reactions Organic Chemistry Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn why terminal alkynes are more. Which Is More Acidic Alkane Alkene Or Alkyne.
From quizlet.com
Naming Alkanes, Alkenes, and Alkynes Diagram Quizlet Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint,. Which Is More Acidic Alkane Alkene Or Alkyne.
From slidetodoc.com
Chapter 7 Reactions of Alkenes and Alkynes 2006 Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and alkynes are more reactive than alkanes. Why are alkynes. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.youtube.com
Year 12 Organic Chemistry Revision Alkanes and alkenes VISUAL Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.numerade.com
SOLVED 4 Label each of the following organic molecules with the most Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and alkynes are more reactive than alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. When it comes. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.numerade.com
⏩SOLVEDAlkyne hydrogens are more acidic than alkene or alkane… Numerade Which Is More Acidic Alkane Alkene Or Alkyne When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Why are alkynes acidic in comparison to alkanes and alkenes? In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. Learn why terminal. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.slideshare.net
Alkane,alkene,alkyne Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Assertion :alkenes and alkynes are more reactive than alkanes. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to. Which Is More Acidic Alkane Alkene Or Alkyne.
From read.cholonautas.edu.pe
What Physical Properties Do Alkanes Alkenes And Alkynes Share Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Assertion :alkenes and. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.slideserve.com
PPT Chapter 9 Alkynes PowerPoint Presentation, free download ID5310048 Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Assertion :alkenes and alkynes are more reactive than alkanes. Why are alkynes acidic in comparison to alkanes and alkenes?. Which Is More Acidic Alkane Alkene Or Alkyne.
From barrygraygillingham.com
A simple introduction to organic chemistry Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Why are alkynes acidic in comparison to alkanes and alkenes? In the. Which Is More Acidic Alkane Alkene Or Alkyne.
From byjus.com
Why is alkynes acidic? Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Assertion :alkenes and alkynes are more reactive than alkanes. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.vrogue.co
Experiment 2 Properties Of Alkanes Alkenes And Alcoho vrogue.co Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Assertion :alkenes and alkynes are more reactive. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.numerade.com
SOLVEDQuestion 28 35285 Terminal alkynes are more acidic than both Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and alkynes are more reactive than alkanes. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.youtube.com
Why are alkynes more acidic than alkenes and alkanes? YouTube Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. In the discussions of acids and bases (chapter 3), we have learned that the. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.youtube.com
Why Alkynes are more acidic than Alkenes and Alkanes ? YouTube Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Assertion :alkenes and alkynes are more reactive than alkanes. Learn why terminal alkynes are. Which Is More Acidic Alkane Alkene Or Alkyne.
From kpu.pressbooks.pub
2.1 Structures of Alkenes Organic Chemistry Which Is More Acidic Alkane Alkene Or Alkyne When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.vrogue.co
Alkanes Alkenes And Alkynes General Molecular Formula vrogue.co Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Assertion :alkenes and alkynes are more reactive than alkanes. In the discussions of. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn why terminal alkynes are more. Which Is More Acidic Alkane Alkene Or Alkyne.
From ar.inspiredpencil.com
Organic Chemistry Functional Groups Alkane Alkene Alkyne Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Why are alkynes acidic in comparison to alkanes and alkenes? Learn how. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.studocu.com
Alkynes more acidic than alkanes or alkenes =X 6 carbon alkyne 2eq Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Assertion :alkenes and alkynes are more reactive than alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher. Which Is More Acidic Alkane Alkene Or Alkyne.
From simp-link.com
C9h18 alkane alkene or alkyne Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and alkynes are more reactive than alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.numerade.com
SOLVED Why are terminal alkynes much more acidic than alkanes? The sp Which Is More Acidic Alkane Alkene Or Alkyne Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to alkanes, alkenes, and. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.solutionspile.com
[Solved] Alkynes are more acidic than alkenes, explain in 1 Which Is More Acidic Alkane Alkene Or Alkyne When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.numerade.com
SOLVED Alkyne hydrogens are more acidic than alkene or alkane Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. Why are alkynes acidic in comparison to alkanes and alkenes? The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.doubtnut.com
Alkane and Alkene Which Is More Acidic Alkane Alkene Or Alkyne When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. The acidity of terminal alkynes is in fact pretty useful from a synthetic. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.chegg.com
Solved the most acidic? Alkanes, Alkenes, Alkynes, Which Is More Acidic Alkane Alkene Or Alkyne In the discussions of acids and bases (chapter 3), we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.studypool.com
SOLUTION Naming alkanes alkenes and alkynes Studypool Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Assertion :alkenes and alkynes are more reactive than alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to. Which Is More Acidic Alkane Alkene Or Alkyne.
From simp-link.com
C9h18 alkane alkene or alkyne Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Why are alkynes acidic in comparison to alkanes and alkenes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Assertion :alkenes and alkynes are more reactive than alkanes.. Which Is More Acidic Alkane Alkene Or Alkyne.
From pakmcqs.com
Out of Alkane, Alkene & Alkyne, which one is more acidic? PakMcqs Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity. Which Is More Acidic Alkane Alkene Or Alkyne.
From brainly.com
Question 1 Alkanes, Alkenes, and Alkynes Complete the table by Which Is More Acidic Alkane Alkene Or Alkyne Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base. Which Is More Acidic Alkane Alkene Or Alkyne.
From www.vrogue.co
Alkane Alkene Aldehyde Alcohol Ketone Acid Amine Ethe vrogue.co Which Is More Acidic Alkane Alkene Or Alkyne The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and. Which Is More Acidic Alkane Alkene Or Alkyne.
From simp-link.com
C9h18 alkane alkene or alkyne Which Is More Acidic Alkane Alkene Or Alkyne Why are alkynes acidic in comparison to alkanes and alkenes? Learn how to compare the acidity of alkane, alkene and alkyne hydrocarbons and other organic compounds. Assertion :alkenes and alkynes are more reactive than alkanes. Learn why terminal alkynes are more acidic than alkanes or alkenes, and how to rank their acidity based on orbital hybridization. The acidity of terminal. Which Is More Acidic Alkane Alkene Or Alkyne.