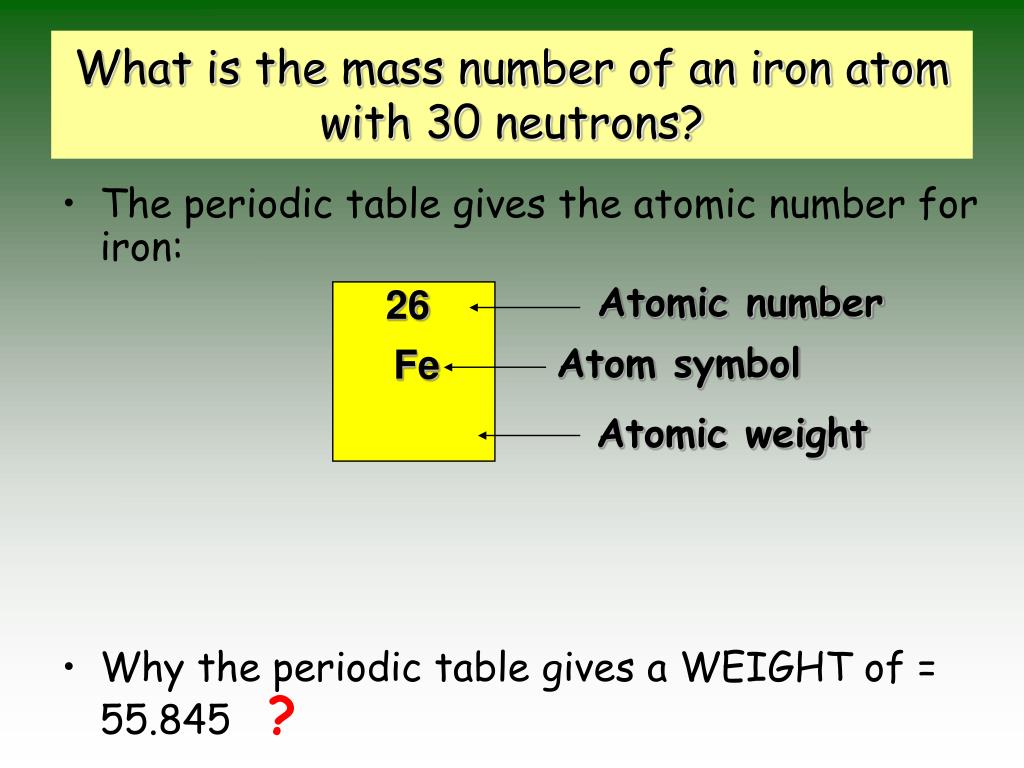
Atomic Mass Of Iron In Amu . Access detailed info on all elements: This calculation gives us the mass of a single atom of an element. Get a free hd image of the periodic. Atomic mass, electron configurations, charges, and more. The atomic mass is the mass of an atom. The unit of measure for mass is the atomic mass unit (amu). As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: View rotating bohr models for all 118 elements. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Atomic mass of iron is 55.845 u. Then came isotopes, the atoms that differ.
from utedzz.blogspot.com
The unit of measure for mass is the atomic mass unit (amu). Get a free hd image of the periodic. Atomic mass of iron is 55.845 u. Atomic mass, electron configurations, charges, and more. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. This calculation gives us the mass of a single atom of an element. The atomic mass is the mass of an atom. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Then came isotopes, the atoms that differ. View rotating bohr models for all 118 elements.
Periodic Table Iron Atomic Mass Periodic Table Timeline
Atomic Mass Of Iron In Amu The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Get a free hd image of the periodic. The unit of measure for mass is the atomic mass unit (amu). Then came isotopes, the atoms that differ. View rotating bohr models for all 118 elements. Access detailed info on all elements: Atomic mass of iron is 55.845 u. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. The atomic mass is the mass of an atom. This calculation gives us the mass of a single atom of an element. Atomic mass, electron configurations, charges, and more. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers:
From ootery.weebly.com
Atomic mass of iron ootery Atomic Mass Of Iron In Amu The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Then came isotopes, the atoms that differ. The atomic mass is the mass of an atom. View rotating bohr models for all 118 elements. This calculation gives us the mass of a single atom of an element. Get a free. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED1. Calculate the mass (in amu) ofa sample of iron(Fe) that Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Access detailed info on all elements: As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Atomic mass of iron is 55.845 u. This calculation gives us the mass of a single atom of an element. Atomic mass, electron configurations, charges, and more. View rotating. Atomic Mass Of Iron In Amu.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Atomic Mass Of Iron In Amu Get a free hd image of the periodic. This calculation gives us the mass of a single atom of an element. Access detailed info on all elements: View rotating bohr models for all 118 elements. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. The atomic mass is the. Atomic Mass Of Iron In Amu.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Atomic Mass Of Iron In Amu This calculation gives us the mass of a single atom of an element. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Get a free hd image of the periodic. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. The. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED Determine the mass percent of iron in FeO to four significant Atomic Mass Of Iron In Amu The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. View rotating bohr models for all 118 elements. Access detailed info on all elements: Then came isotopes, the atoms that differ. The unit of measure for mass is the atomic mass. Atomic Mass Of Iron In Amu.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Atomic mass, electron configurations, charges, and more. Atomic mass of iron is 55.845 u. Get a free hd image of the periodic. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: The atomic mass or relative isotopic mass refers to the mass of a. Atomic Mass Of Iron In Amu.
From www.youtube.com
Relative Atomic Mass and Atomic Mass Unit (amu) Fundamental of Atomic Mass Of Iron In Amu As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Get a free hd image of the periodic. This calculation gives us the mass of a single atom of an element. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Atomic. Atomic Mass Of Iron In Amu.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Atomic Mass Of Iron In Amu Atomic mass, electron configurations, charges, and more. The unit of measure for mass is the atomic mass unit (amu). As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Atomic mass of iron is 55.845 u. The atomic mass is the mass of an atom. View rotating bohr models for all 118. Atomic Mass Of Iron In Amu.
From www.researchgate.net
The duration of 3 meteor trails of different atomic masses 8, 30 and Atomic Mass Of Iron In Amu Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. This calculation gives us the mass of a single atom of an element. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Get a free hd image of the periodic. The unit of measure for mass. Atomic Mass Of Iron In Amu.
From www.youtube.com
ATOMIC MASS OF IRON YouTube Atomic Mass Of Iron In Amu Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: This calculation gives us the mass of a single atom of an element. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Then came isotopes, the atoms that differ. Get a free hd image of the periodic. Atomic. Atomic Mass Of Iron In Amu.
From www.vectorstock.com
Flashcard of iron with atomic mass Royalty Free Vector Image Atomic Mass Of Iron In Amu The unit of measure for mass is the atomic mass unit (amu). This calculation gives us the mass of a single atom of an element. Then came isotopes, the atoms that differ. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Get a free hd image of the periodic.. Atomic Mass Of Iron In Amu.
From www.periodictableprintable.com
Iron Periodic Table Electrons 2023 Periodic Table Printable Atomic Mass Of Iron In Amu Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. This calculation gives us the mass of a single atom of an element. Access detailed info on all elements: The unit of measure for mass. Atomic Mass Of Iron In Amu.
From zaiden-jolpblogmoreno.blogspot.com
Atomic Mass of Iron Atomic Mass Of Iron In Amu The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. View rotating bohr models for all 118 elements. This calculation gives us the mass of a single atom of an element. Then came isotopes, the atoms that differ. Atomic mass of iron is 55.845 u. Get a free hd image. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED The average atomic mass of iron is 55.847 amu. Knowing how Atomic Mass Of Iron In Amu The atomic mass is the mass of an atom. Get a free hd image of the periodic. This calculation gives us the mass of a single atom of an element. View rotating bohr models for all 118 elements. Atomic mass of iron is 55.845 u. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: The atomic. Atomic Mass Of Iron In Amu.
From gbu-presnenskij.ru
SOLVED Calculate The Average Atomic Mass Of Iron Using The, 43 OFF Atomic Mass Of Iron In Amu This calculation gives us the mass of a single atom of an element. Atomic mass, electron configurations, charges, and more. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Then came isotopes, the atoms that differ. Access detailed info on all elements: The unit of measure for mass is. Atomic Mass Of Iron In Amu.
From slideplayer.com
Review of Atomic Structure ppt download Atomic Mass Of Iron In Amu This calculation gives us the mass of a single atom of an element. Atomic mass, electron configurations, charges, and more. The unit of measure for mass is the atomic mass unit (amu). Then came isotopes, the atoms that differ. The atomic mass is the mass of an atom. Atomic mass of iron is 55.845 u. View rotating bohr models for. Atomic Mass Of Iron In Amu.
From www.alamy.com
Iron chemical element. Chemical symbol with atomic number and atomic Atomic Mass Of Iron In Amu Atomic mass of iron is 55.845 u. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: This calculation gives us the mass of a single atom of an element. Get a free hd image of the periodic. The unit of measure for mass is the atomic mass unit (amu). Access detailed. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED The average atomic mass of iron is 55.847 amu. Knowing how Atomic Mass Of Iron In Amu View rotating bohr models for all 118 elements. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Then came isotopes, the atoms that differ. Get a free hd image of the periodic. Access detailed info on all elements: This calculation. Atomic Mass Of Iron In Amu.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Atomic Mass Of Iron In Amu The atomic mass is the mass of an atom. The unit of measure for mass is the atomic mass unit (amu). Access detailed info on all elements: This calculation gives us the mass of a single atom of an element. Then came isotopes, the atoms that differ. The atomic mass or relative isotopic mass refers to the mass of a. Atomic Mass Of Iron In Amu.
From socratic.org
Why don't any of the isotopes of natural iron have the atomic mass of Atomic Mass Of Iron In Amu Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: Then came isotopes, the atoms that differ. This calculation gives us the mass of a single atom of an element. The unit of measure for mass is the atomic mass unit (amu). The atomic mass is the mass of an atom. The atomic mass or relative isotopic. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED Calculate the average atomic mass of iron using the isotopes in Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Access detailed info on all elements: The atomic mass is the mass of an atom. View rotating bohr models for all 118 elements. The unit of measure for mass is the atomic mass unit (amu). This calculation gives us the mass of a single atom of an element. The atomic mass or relative. Atomic Mass Of Iron In Amu.
From tankhrom.weebly.com
Iron atomic number tankhrom Atomic Mass Of Iron In Amu This calculation gives us the mass of a single atom of an element. Access detailed info on all elements: The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. The unit of measure for mass is the atomic mass unit (amu).. Atomic Mass Of Iron In Amu.
From utedzz.blogspot.com
Periodic Table Iron Atomic Mass Periodic Table Timeline Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Atomic mass, electron configurations, charges, and more. The atomic mass is the mass of an atom. Access detailed info on all elements: This calculation gives us the mass of a single atom of an element. Atomic mass of iron is 55.845 u. The unit of measure for mass is the atomic mass unit. Atomic Mass Of Iron In Amu.
From www.scribd.com
Atomic Masses of First 30 Elements PDF Chemical Elements Iron Atomic Mass Of Iron In Amu View rotating bohr models for all 118 elements. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. The unit of measure for mass is the atomic mass unit (amu). The atomic mass is the mass of an atom. This calculation gives us the mass of a single atom of. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED Iron has an atomic mass of 55.845 amu The mass numbers and Atomic Mass Of Iron In Amu Atomic mass of iron is 55.845 u. The unit of measure for mass is the atomic mass unit (amu). Access detailed info on all elements: Get a free hd image of the periodic. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Then came isotopes, the atoms that differ. The atomic. Atomic Mass Of Iron In Amu.
From www.numerade.com
Determine the atomic mass of iron, given the following information Atomic Mass Of Iron In Amu As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: This calculation gives us the mass of a single atom of an element. View rotating bohr models for all 118 elements. Then came isotopes, the atoms that differ. The atomic mass is the mass of an atom. The unit of measure for. Atomic Mass Of Iron In Amu.
From www.adda247.com
Atomic Mass of Elements 1 to 30 with Symbols PDF Download Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: View rotating bohr models for all 118 elements. This calculation gives us the mass of a single atom of an element. Access detailed info on all elements: Get a free hd image of the periodic.. Atomic Mass Of Iron In Amu.
From syatillakmk.blogspot.com
SimplyChemistry C1 1.2RELATIVE ATOMIC MASS (R.A.M) Atomic Mass Of Iron In Amu Get a free hd image of the periodic. Atomic mass of iron is 55.845 u. The atomic mass is the mass of an atom. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: This calculation gives us the mass of a single atom of an element. The atomic mass or relative isotopic mass refers to the. Atomic Mass Of Iron In Amu.
From www.chegg.com
Solved Type numbers in the boxes. The iron isotopes in an Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Atomic mass, electron configurations, charges, and more. The unit of measure for mass is the atomic mass unit (amu). Get a free hd image of the periodic. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. View rotating bohr models for all. Atomic Mass Of Iron In Amu.
From www.slideserve.com
PPT Chapter 14 Atoms and the Periodic Table PowerPoint Presentation Atomic Mass Of Iron In Amu Atomic mass of iron is 55.845 u. Then came isotopes, the atoms that differ. The unit of measure for mass is the atomic mass unit (amu). Get a free hd image of the periodic. Access detailed info on all elements: As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: The atomic. Atomic Mass Of Iron In Amu.
From inspiritvr.com
Atomic Mass Unit Study Guide Inspirit Atomic Mass Of Iron In Amu The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Atomic mass of iron is 55.845 u. This calculation gives us the mass of a single atom of an element. The unit of measure for mass is the atomic mass unit. Atomic Mass Of Iron In Amu.
From www.numerade.com
SOLVED Using the average atomic mass, calculate the mass in grams of Atomic Mass Of Iron In Amu Then came isotopes, the atoms that differ. Atomic mass, electron configurations, charges, and more. As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: Access detailed info on all elements: The unit of measure for mass is the atomic mass unit (amu). The atomic mass is the mass of an atom. Atomic. Atomic Mass Of Iron In Amu.
From www.youtube.com
How to Find the Average Atomic Mass of Iron YouTube Atomic Mass Of Iron In Amu As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: This calculation gives us the mass of a single atom of an element. Then came isotopes, the atoms that differ. Access detailed info on all elements: Atomic mass of iron is 55.845 u. View rotating bohr models for all 118 elements. Atomic. Atomic Mass Of Iron In Amu.
From www.vrogue.co
Iron Chemical Element Periodic Table Science Symbol S vrogue.co Atomic Mass Of Iron In Amu As we saw earlier, it is convenient to use a reference unit when dealing with such small numbers: This calculation gives us the mass of a single atom of an element. Then came isotopes, the atoms that differ. Get a free hd image of the periodic. The atomic mass or relative isotopic mass refers to the mass of a single. Atomic Mass Of Iron In Amu.
From mymumbaipost.in
What is Atomic Mass of Iron? Detailed Answer Mymumbaipost Atomic Mass Of Iron In Amu The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to. Atomic mass of iron is 55.845 u. The unit of measure for mass is the atomic mass unit (amu). Then came isotopes, the atoms that differ. As we saw earlier, it. Atomic Mass Of Iron In Amu.