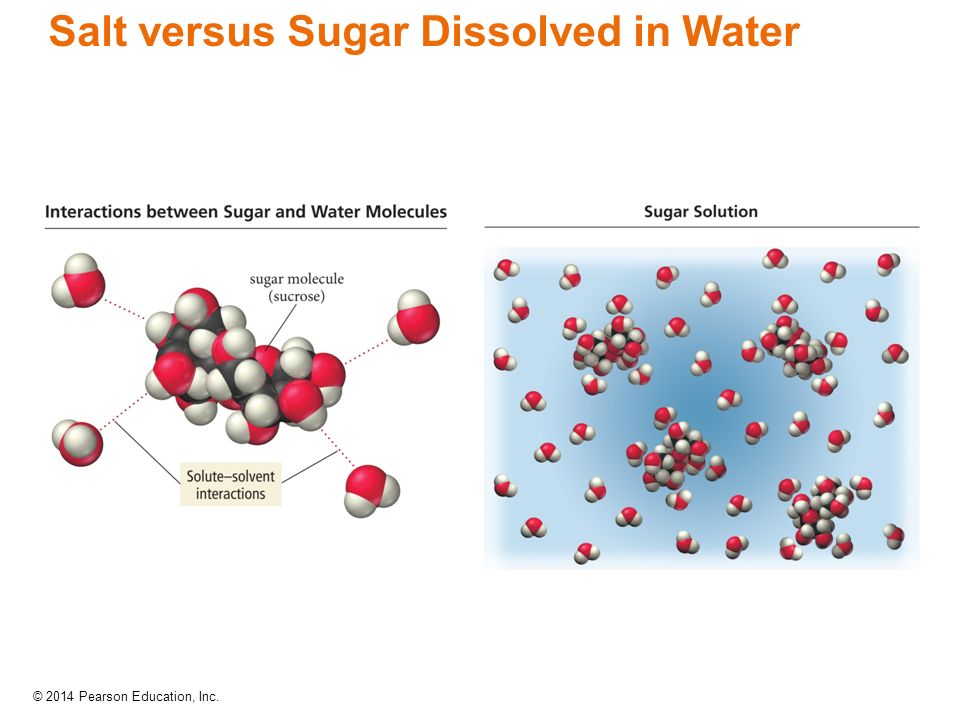
Salt Vs Sugar Dissolving In Water . Or they might think that. What happens when sugar and salt are added to water? Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Students may think that sugar is made of ionic bonds like salt. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. The weak bonds that form between. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water.
from mungfali.com
Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” What happens when sugar and salt are added to water? The weak bonds that form between. Or they might think that. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Students may think that sugar is made of ionic bonds like salt. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water.
Dissolving Salt In Water Diagram
Salt Vs Sugar Dissolving In Water There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. What happens when sugar and salt are added to water? Students may think that sugar is made of ionic bonds like salt. Or they might think that. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. The weak bonds that form between. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules.
From rayb78.github.io
Solubility In Water Chart Salt Vs Sugar Dissolving In Water Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Or they might think that. The weak bonds that form between. Knowing what you do about the polarity of water, why do you think water dissolves sugar? What happens when sugar and salt are added to water? This formative assessment looks at two household chemicals (table. Salt Vs Sugar Dissolving In Water.
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC Salt Vs Sugar Dissolving In Water The weak bonds that form between. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Or they might think that. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar” formative assessment explores students’ thinking about the question “how. Salt Vs Sugar Dissolving In Water.
From stock.adobe.com
Ionic vs. Covalent Compounds in an aqueous solution. Sugar and Salt Salt Vs Sugar Dissolving In Water This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Or they might think that. Sugar. Salt Vs Sugar Dissolving In Water.
From wt.kimiq.com
Salt Water Chemical Formula Water Ionizer Salt Vs Sugar Dissolving In Water There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar. Salt Vs Sugar Dissolving In Water.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water Salt Vs Sugar Dissolving In Water Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Or they might think that. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. What happens when sugar and salt are added to water? Knowing what you do. Salt Vs Sugar Dissolving In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Salt Vs Sugar Dissolving In Water Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Students may think that sugar is made of ionic bonds like salt. What happens when sugar and salt are added to water? This formative assessment looks at two household chemicals. Salt Vs Sugar Dissolving In Water.
From www.ck12.org
to CK12 Foundation CK12 Foundation Salt Vs Sugar Dissolving In Water Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Or they might think that. What happens when sugar and salt are added to water? Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Students may think that sugar is made of ionic bonds like salt. This formative. Salt Vs Sugar Dissolving In Water.
From www.researchgate.net
Illustration of dilution of sugar/salt in the aqueous solution (a) and Salt Vs Sugar Dissolving In Water Knowing what you do about the polarity of water, why do you think water dissolves sugar? There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules.. Salt Vs Sugar Dissolving In Water.
From www.youtube.com
Pop Up Science Sugar and Water YouTube Salt Vs Sugar Dissolving In Water Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between. Students may think that sugar is made of ionic bonds like salt. There is no. Salt Vs Sugar Dissolving In Water.
From www.thoughtco.com
Dissolving Sugar in Water Chemical or Physical Change? Salt Vs Sugar Dissolving In Water What happens when sugar and salt are added to water? Or they might think that. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Students may think that sugar is made of ionic bonds. Salt Vs Sugar Dissolving In Water.
From www.shutterstock.com
Ilustración del producto químico. La solubilidad vector de stock Salt Vs Sugar Dissolving In Water Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. What happens when sugar and salt are added to water? The weak bonds that form between. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” This formative assessment looks at two household. Salt Vs Sugar Dissolving In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Salt Vs Sugar Dissolving In Water Or they might think that. Students may think that sugar is made of ionic bonds like salt. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and. Salt Vs Sugar Dissolving In Water.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Salt Vs Sugar Dissolving In Water Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Or they might think that. Sugar dissolves in. Salt Vs Sugar Dissolving In Water.
From brightideas.houstontx.gov
Create A Visual Model Of An Ionic Substance (salt) Dissolving In Water Salt Vs Sugar Dissolving In Water What happens when sugar and salt are added to water? Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar dissolves in water because. Salt Vs Sugar Dissolving In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Salt Vs Sugar Dissolving In Water Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Students may think that sugar is made of ionic bonds like salt. Or they might think that. The weak bonds that form. Salt Vs Sugar Dissolving In Water.
From wiringdiagrambig.z19.web.core.windows.net
Diagram Of Sugar Dissolving In Water Salt Vs Sugar Dissolving In Water The weak bonds that form between. Knowing what you do about the polarity of water, why do you think water dissolves sugar? Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Or they might think that. There is no obvious difference between the amount of salt that dissolves in the hot water. Salt Vs Sugar Dissolving In Water.
From www.ck12.org
Solubility CK12 Foundation Salt Vs Sugar Dissolving In Water Students may think that sugar is made of ionic bonds like salt. The weak bonds that form between. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar has. Salt Vs Sugar Dissolving In Water.
From www.visionlearning.org
Properties of Solids Chemistry Visionlearning Salt Vs Sugar Dissolving In Water Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Or they might think that. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking. Salt Vs Sugar Dissolving In Water.
From www.youtube.com
WCLN Osmosis water sugar solution Biology YouTube Salt Vs Sugar Dissolving In Water Students may think that sugar is made of ionic bonds like salt. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. The weak bonds that form between. What happens when sugar and salt are added to water? Sugar dissolves in water because energy is given off when the. Salt Vs Sugar Dissolving In Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water Salt Vs Sugar Dissolving In Water Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Students may think that sugar is made of ionic bonds like salt. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. This formative assessment looks at two household chemicals (table salt and sugar) and. Salt Vs Sugar Dissolving In Water.
From www.youtube.com
which substance dissolve first in water sugar or salt? YouTube Salt Vs Sugar Dissolving In Water This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: The weak bonds that form between. Students may think that sugar is made of ionic bonds like salt. Knowing what you do. Salt Vs Sugar Dissolving In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 Salt Vs Sugar Dissolving In Water This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Students may think that sugar is made of ionic bonds like salt. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Pour in sugar, shake in salt, and evaporate water to. Salt Vs Sugar Dissolving In Water.
From www.chegg.com
Solved [5 Points] 4. With reference to the solubility of Salt Vs Sugar Dissolving In Water The weak bonds that form between. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Students may think that sugar is made of ionic bonds like salt. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” There is no obvious difference between the amount of salt that dissolves in the. Salt Vs Sugar Dissolving In Water.
From www.slideshare.net
Solubility (a physical property) (Teach) Salt Vs Sugar Dissolving In Water Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar” formative assessment explores students’ thinking about the. Salt Vs Sugar Dissolving In Water.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Salt Vs Sugar Dissolving In Water The weak bonds that form between. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Knowing what you do about the polarity. Salt Vs Sugar Dissolving In Water.
From ar.inspiredpencil.com
Sugar And Water Solution Salt Vs Sugar Dissolving In Water There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Or they might think that. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Students may think that sugar is made of ionic bonds. Salt Vs Sugar Dissolving In Water.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Salt Vs Sugar Dissolving In Water What happens when sugar and salt are added to water? There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar dissolves in water because energy is given off when the slightly. Salt Vs Sugar Dissolving In Water.
From www.slideserve.com
PPT Chapter 10 Chemical Reactivity PowerPoint Presentation, free Salt Vs Sugar Dissolving In Water Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Students may think that sugar is made of ionic bonds like salt. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: There is no obvious difference between the amount of salt that dissolves in the hot water compared. Salt Vs Sugar Dissolving In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Salt Vs Sugar Dissolving In Water Or they might think that. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” The weak bonds that form between. Pour in sugar, shake in salt, and evaporate water to see. Salt Vs Sugar Dissolving In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Salt Vs Sugar Dissolving In Water Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar” formative assessment explores students’ thinking about the question “how does structure influence reactivity?” Or they might think that. Pour in sugar,. Salt Vs Sugar Dissolving In Water.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Salt Vs Sugar Dissolving In Water Knowing what you do about the polarity of water, why do you think water dissolves sugar? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: The weak bonds that form between. Students may think that sugar is made of ionic bonds like salt. This formative assessment looks at two household chemicals (table salt and sugar). Salt Vs Sugar Dissolving In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Salt Vs Sugar Dissolving In Water What happens when sugar and salt are added to water? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Knowing what you do about the polarity of water, why do you think water dissolves sugar? This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how. Salt Vs Sugar Dissolving In Water.
From stock.adobe.com
Vecteur Stock Chemical illustration. The solubility of sugar and water Salt Vs Sugar Dissolving In Water Knowing what you do about the polarity of water, why do you think water dissolves sugar? What happens when sugar and salt are added to water? Students may think that sugar is made of ionic bonds like salt. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Pour in sugar, shake in salt, and evaporate. Salt Vs Sugar Dissolving In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 Salt Vs Sugar Dissolving In Water What happens when sugar and salt are added to water? This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Knowing what you do about the polarity of water, why do you. Salt Vs Sugar Dissolving In Water.
From mungfali.com
Dissolving Salt In Water Diagram Salt Vs Sugar Dissolving In Water There is no obvious difference between the amount of salt that dissolves in the hot water compared to the cold water. What happens when sugar and salt are added to water? Or they might think that. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water.. Salt Vs Sugar Dissolving In Water.