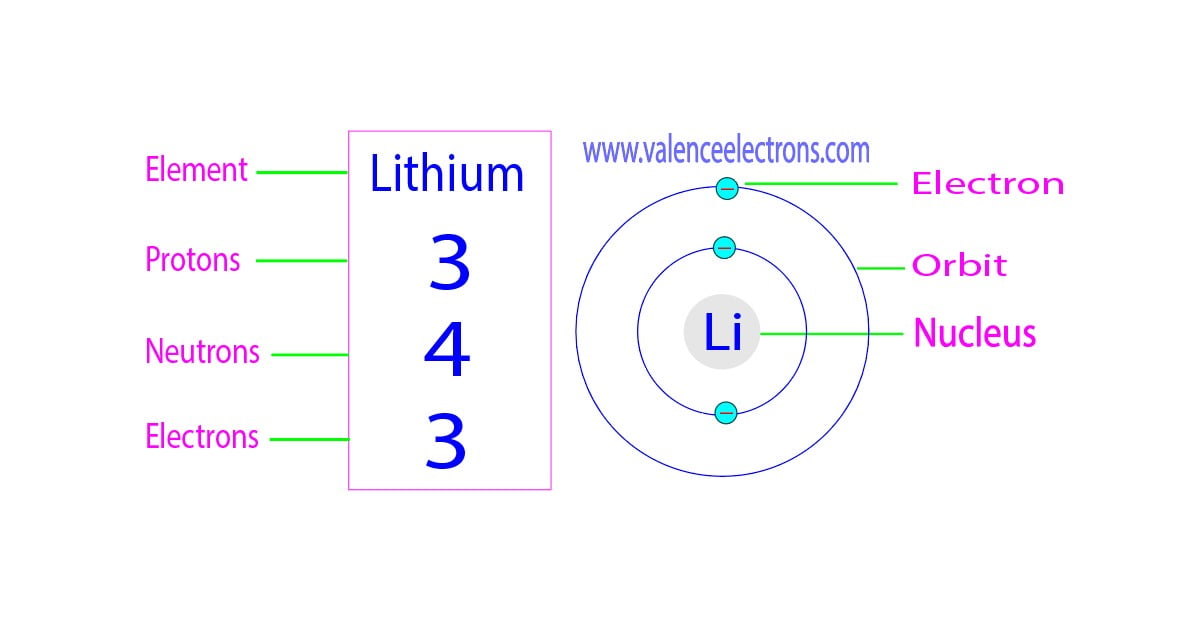
Lead Electrons Neutrons Protons . This is approximately the sum of the number of protons and neutrons in the nucleus. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Total number of protons in the nucleus is called the atomic number of the atom. Electrons are found outside the nucleus. Determine the number of protons and. It has an atomic weight of 207.2 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Describe the locations, charges, and masses of the three main subatomic particles. Where more than one isotope exists, the value given is the. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Protons and neutrons are found in the nucleus, the dense region at the center of an atom.
from valenceelectrons.com
Determine the number of protons and. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Total number of protons in the nucleus is called the atomic number of the atom. Describe the locations, charges, and masses of the three main subatomic particles. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Where more than one isotope exists, the value given is the. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. It has an atomic weight of 207.2 and a mass. This is approximately the sum of the number of protons and neutrons in the nucleus.
How to find the Protons, Neutrons and Electrons for Lead?
Lead Electrons Neutrons Protons Total number of protons in the nucleus is called the atomic number of the atom. It has an atomic weight of 207.2 and a mass. Electrons are found outside the nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the. Describe the locations, charges, and masses of the three main subatomic particles. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Determine the number of protons and. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Total number of protons in the nucleus is called the atomic number of the atom. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Electrons Neutrons Protons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Describe the locations, charges, and masses of the three main subatomic particles. This is approximately the sum of the number of protons and. Lead Electrons Neutrons Protons.
From www.slideshare.net
atoms Lead Electrons Neutrons Protons Total number of protons in the nucleus is called the atomic number of the atom. Electrons are found outside the nucleus. This is approximately the sum of the number of protons and neutrons in the nucleus. Determine the number of protons and. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus.. Lead Electrons Neutrons Protons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead Electrons Neutrons Protons Total number of protons in the nucleus is called the atomic number of the atom. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Describe the locations, charges, and masses of. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons It has an atomic weight of 207.2 and a mass. Describe the locations, charges, and masses of the three main subatomic particles. Electrons are found outside the nucleus. This is approximately the sum of the number of protons and neutrons in the nucleus. Protons and neutrons are found in the nucleus, the dense region at the center of an atom.. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. It has an atomic weight of 207.2 and a mass. This is approximately the sum of the number of protons and neutrons in the nucleus.. Lead Electrons Neutrons Protons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Lead Electrons Neutrons Protons Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. This is approximately the sum of the number of protons and neutrons in the nucleus. It has an atomic weight of 207.2 and a mass.. Lead Electrons Neutrons Protons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Mercury (Hg, Hg2+) Lead Electrons Neutrons Protons Where more than one isotope exists, the value given is the. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and. It has an atomic weight of 207.2 and a mass. Total number. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. It has an atomic weight of 207.2 and a mass. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more. Lead Electrons Neutrons Protons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Pt (Platinum Lead Electrons Neutrons Protons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Total number of protons in the nucleus is called the atomic number of the atom. Determine the number of protons and. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. This is approximately the sum of. Lead Electrons Neutrons Protons.
From valenceelectrons.com
How many protons, neutrons and electrons does potassium have? Lead Electrons Neutrons Protons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Electrons are found outside the nucleus. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than. Lead Electrons Neutrons Protons.
From www.broadlearnings.com
Atomic Structure Broad Learnings Lead Electrons Neutrons Protons Electrons are found outside the nucleus. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Describe the locations, charges, and masses of the three main subatomic particles. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and. Lead Electrons Neutrons Protons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Lead? Lead Electrons Neutrons Protons This is approximately the sum of the number of protons and neutrons in the nucleus. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Describe the locations, charges, and masses of the three main subatomic particles. Total number of protons in the nucleus is called the atomic number of the atom.. Lead Electrons Neutrons Protons.
From www.expii.com
Electrons — Structure & Properties Expii Lead Electrons Neutrons Protons It has an atomic weight of 207.2 and a mass. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons,. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Determine the number of protons and. Electrons are found outside the nucleus. Where more than one isotope exists, the value given is the. Lead. Lead Electrons Neutrons Protons.
From ar.inspiredpencil.com
Electron Proton Neutron Lead Electrons Neutrons Protons Describe the locations, charges, and masses of the three main subatomic particles. It has an atomic weight of 207.2 and a mass. This is approximately the sum of the number of protons and neutrons in the nucleus. Electrons are found outside the nucleus. Determine the number of protons and. Total number of protons in the nucleus is called the atomic. Lead Electrons Neutrons Protons.
From slidetodoc.com
Protons neutrons electrons Pencil Zoom The lead of Lead Electrons Neutrons Protons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom. Determine the number of protons and. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Protons and. Lead Electrons Neutrons Protons.
From www.expii.com
Neutrons — Structure & Properties Expii Lead Electrons Neutrons Protons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Where more than one isotope exists, the value. Lead Electrons Neutrons Protons.
From www.periodictableprintable.com
Lead Periodic Table Protons Neutrons And Electrons 2024 Periodic Lead Electrons Neutrons Protons It has an atomic weight of 207.2 and a mass. This is approximately the sum of the number of protons and neutrons in the nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Electrons are found outside the nucleus. Total number of protons in the nucleus is called. Lead Electrons Neutrons Protons.
From ar.inspiredpencil.com
Proton Neutron Electron Periodic Table Lead Electrons Neutrons Protons Electrons are found outside the nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Where more than one isotope exists, the value given is the. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. This is approximately the sum of the number of. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Describe the locations, charges, and masses of the three main subatomic particles. Electrons are found outside the nucleus. It has an atomic weight of 207.2 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table. Lead Electrons Neutrons Protons.
From exoxpojqw.blob.core.windows.net
Lead 214 Protons Neutrons Electrons at Heather Doss blog Lead Electrons Neutrons Protons Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Where more than one isotope exists, the value given is the. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Total number of protons in the nucleus is called the atomic. Lead Electrons Neutrons Protons.
From www.teachoo.com
Neutron Discovery, Difference and more Teachoo Concepts Lead Electrons Neutrons Protons Where more than one isotope exists, the value given is the. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Total number of protons in the nucleus is called the atomic number of the atom. Lead is a chemical element with atomic number 82 which means there are 82. Lead Electrons Neutrons Protons.
From azchemistry.com
Proton, Electron, Neutron Definition Formula Application Lead Electrons Neutrons Protons It has an atomic weight of 207.2 and a mass. Describe the locations, charges, and masses of the three main subatomic particles. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons,. Lead Electrons Neutrons Protons.
From www.dreamstime.com
Lead stock illustration. Illustration of atomic, symbol 175796461 Lead Electrons Neutrons Protons This is approximately the sum of the number of protons and neutrons in the nucleus. It has an atomic weight of 207.2 and a mass. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number. Lead Electrons Neutrons Protons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Electrons Neutrons Protons Where more than one isotope exists, the value given is the. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. This is approximately the sum of the number of protons and neutrons in the nucleus. Total. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Describe the locations, charges, and masses of the three main subatomic particles. Electrons are found outside the nucleus. Lead is a chemical element with atomic number. Lead Electrons Neutrons Protons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Helium (He Lead Electrons Neutrons Protons Describe the locations, charges, and masses of the three main subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Where more than one isotope exists, the value given is the. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Electrons are. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons Total number of protons in the nucleus is called the atomic number of the atom. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Determine the number of protons and. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82.. Lead Electrons Neutrons Protons.
From www.sliderbase.com
Isotopes. Lead Electrons Neutrons Protons Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Electrons are found outside the nucleus. Determine the number of protons and. Where more than one isotope exists, the value given is the. This is approximately the sum of the number of protons and neutrons in the nucleus. Total number. Lead Electrons Neutrons Protons.
From es.vecteezy.com
modelo de estructura de átomo, núcleo de protones y neutrones Lead Electrons Neutrons Protons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Describe. Lead Electrons Neutrons Protons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electrons Neutrons Protons Describe the locations, charges, and masses of the three main subatomic particles. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. This is approximately the sum of the number of protons and neutrons in the nucleus. Lead is a chemical element with atomic number 82 which means there are. Lead Electrons Neutrons Protons.
From exoxpojqw.blob.core.windows.net
Lead 214 Protons Neutrons Electrons at Heather Doss blog Lead Electrons Neutrons Protons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Protons and neutrons are found in the nucleus,. Lead Electrons Neutrons Protons.
From www.coursehero.com
[Solved] Symbol 35 CI 17 Protons 26 82 Neutrons 30 66 Electrons 49 86 Lead Electrons Neutrons Protons Determine the number of protons and. This is approximately the sum of the number of protons and neutrons in the nucleus. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. In this. Lead Electrons Neutrons Protons.
From mungfali.com
Protons Neutrons And Electrons Diagram Lead Electrons Neutrons Protons Where more than one isotope exists, the value given is the. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Lead is the 82nd element in the periodic table and has a symbol of pb and atomic number of 82. Electrons are found outside the nucleus. Total number of protons in the nucleus is. Lead Electrons Neutrons Protons.
From mungfali.com
Protons Neutrons And Electrons Diagram Lead Electrons Neutrons Protons Describe the locations, charges, and masses of the three main subatomic particles. Electrons are found outside the nucleus. Lead is a chemical element with atomic number 82 which means there are 82 protons in its nucleus. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Determine the number of protons and. 112. Lead Electrons Neutrons Protons.