Buffer And Titration Practice Problems . In order to understand buffers and buffer problems we must be proficient with the following. A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Must know the conjugate a/b concept to be. Consider the titration of 25.00 ml of 0.1000 m. The total molarity of acid and. A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the.
from studylib.net
A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Must know the conjugate a/b concept to be. Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is known as a titration curve. The total molarity of acid and. In order to understand buffers and buffer problems we must be proficient with the following. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution).
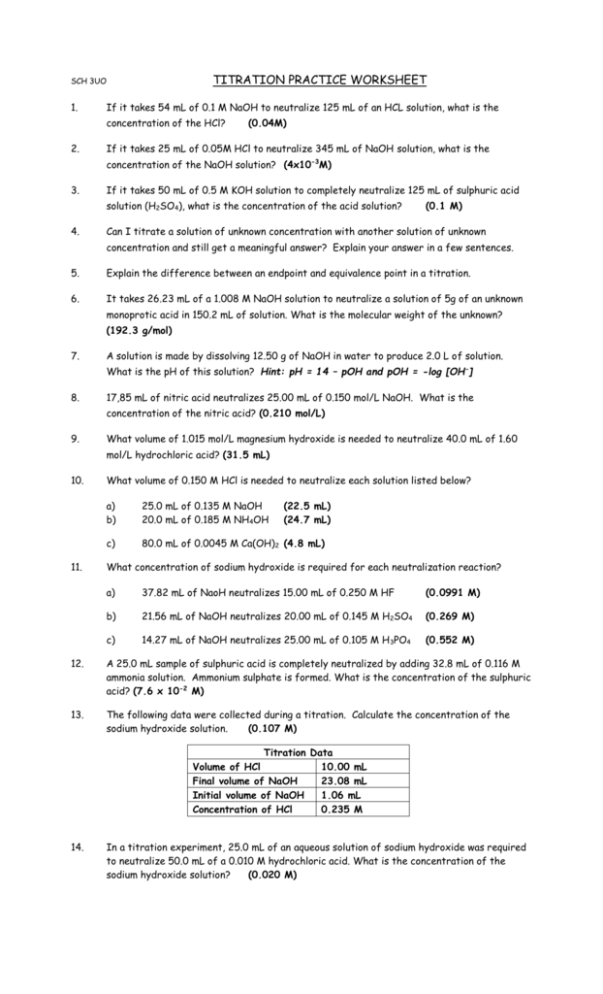
Titration Practice Worksheet
Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. The total molarity of acid and. A plot of ph versus volume of base added gives what is known as a titration curve. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient with the following. Must know the conjugate a/b concept to be. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Consider the titration of 25.00 ml of 0.1000 m.
From theedge.com.hk
Chemistry How To Titration The Edge Buffer And Titration Practice Problems Must know the conjugate a/b concept to be. A plot of ph versus volume of base added gives what is known as a titration curve. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The total molarity of acid and. Consider the titration of 25.00 ml of 0.1000 m.. Buffer And Titration Practice Problems.
From www.studocu.com
Handout Ch 04, Titration Practice Problems Titration Practice Buffer And Titration Practice Problems The total molarity of acid and. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A plot of ph versus volume. Buffer And Titration Practice Problems.
From www.chegg.com
Solved Practice Problems for AcidBase titration. Find the Buffer And Titration Practice Problems A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. Consider the titration of 25.00 ml of 0.1000 m. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In order to understand buffers and buffer problems we must be. Buffer And Titration Practice Problems.
From www.studypool.com
SOLUTION Chemical engineering review ph of monoprotic acid ph of Buffer And Titration Practice Problems In order to understand buffers and buffer problems we must be proficient with the following. Must know the conjugate a/b concept to be. A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m. The total molarity of acid and. Explain how a buffer solution. Buffer And Titration Practice Problems.
From www.chegg.com
Solved Buffer problems and preliminary titration stuff... Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Must know the conjugate a/b concept to be. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient. Buffer And Titration Practice Problems.
From www.scribd.com
lec. 4 buffer solution and titration PDF Chemistry Titration Buffer And Titration Practice Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The total molarity of acid and. A plot of ph versus volume. Buffer And Titration Practice Problems.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Buffer And Titration Practice Problems Consider the titration of 25.00 ml of 0.1000 m. In order to understand buffers and buffer problems we must be proficient with the following. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize. Buffer And Titration Practice Problems.
From www.vrogue.co
Acid Base Titration Problems Basic Introduction Calcu vrogue.co Buffer And Titration Practice Problems A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. A plot of ph versus volume of base added gives what is known as a titration curve. Explain how a. Buffer And Titration Practice Problems.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Buffer And Titration Practice Problems In order to understand buffers and buffer problems we must be proficient with the following. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. In the titration of 80.0. Buffer And Titration Practice Problems.
From www.youtube.com
Challenging Buffer Problems worked out YouTube Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient with the following. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or. Buffer And Titration Practice Problems.
From lessonmagicchampart.z14.web.core.windows.net
Titration Math Practice Problems Buffer And Titration Practice Problems Consider the titration of 25.00 ml of 0.1000 m. The total molarity of acid and. A plot of ph versus volume of base added gives what is known as a titration curve. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Explain how a buffer solution manages to stabilize. Buffer And Titration Practice Problems.
From www.studocu.com
Test3 ch17b BufferTitrationEquilibrium Practice Problems General Buffer And Titration Practice Problems A plot of ph versus volume of base added gives what is known as a titration curve. The total molarity of acid and. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. In the titration of 80.0 ml of 0.150. Buffer And Titration Practice Problems.
From jetpaper.web.fc2.com
solving titration problems Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. In order to understand buffers and buffer problems we must be proficient. Buffer And Titration Practice Problems.
From ar.inspiredpencil.com
Acid Base Titration Experiment Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with. Buffer And Titration Practice Problems.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Buffer And Titration Practice Problems Consider the titration of 25.00 ml of 0.1000 m. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient with the following. In this section, we will see how to perform calculations to predict the ph at any. Buffer And Titration Practice Problems.
From www.studocu.com
Buffer PracticeKey Practice Worksheet key Buffer Practice Problems Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In order to understand buffers and buffer problems we must be proficient with the following. A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. A plot of ph versus. Buffer And Titration Practice Problems.
From www.studocu.com
Titration Curve Practice Problems MATH + SCIENCE INITIATIVE Titration Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A beaker with 100.0 ml of an acetic acid buffer with a. Buffer And Titration Practice Problems.
From www.vrogue.co
Acid Base Titration Problems Basic Introduction Calcu vrogue.co Buffer And Titration Practice Problems A beaker with 100.0 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. Must know the conjugate a/b concept to be. The total molarity of acid and. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. A plot of ph versus volume. Buffer And Titration Practice Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Buffer And Titration Practice Problems Consider the titration of 25.00 ml of 0.1000 m. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Must know the conjugate a/b concept to be. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl,. Buffer And Titration Practice Problems.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A beaker with 100.0 ml of an acetic acid buffer with a. Buffer And Titration Practice Problems.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient with the following. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). A plot of ph versus. Buffer And Titration Practice Problems.
From www.studypool.com
SOLUTION Chemical engineering review ph of monoprotic acid ph of Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In order to understand buffers and buffer problems we must be proficient with the following. A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to. Buffer And Titration Practice Problems.
From www.madebyteachers.com
Solution and Titration Stoichiometry a Chemistry Worksheet Made By Buffer And Titration Practice Problems A plot of ph versus volume of base added gives what is known as a titration curve. The total molarity of acid and. Must know the conjugate a/b concept to be. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In the titration of 80.0 ml of 0.150 m. Buffer And Titration Practice Problems.
From www.studocu.com
Back titration hvuccccfchcinm n hvhjvhivi;vhvh Backtitration Buffer And Titration Practice Problems A plot of ph versus volume of base added gives what is known as a titration curve. Must know the conjugate a/b concept to be. In order to understand buffers and buffer problems we must be proficient with the following. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each.. Buffer And Titration Practice Problems.
From criticalthinking.cloud
buffer solution questions a level chemistry Buffer And Titration Practice Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is known as a titration curve. The total molarity of acid and. In this section, we will see how to. Buffer And Titration Practice Problems.
From www.youtube.com
Practice Buffer Action problems YouTube Buffer And Titration Practice Problems Must know the conjugate a/b concept to be. A plot of ph versus volume of base added gives what is known as a titration curve. In order to understand buffers and buffer problems we must be proficient with the following. The total molarity of acid and. Consider the titration of 25.00 ml of 0.1000 m. Explain how a buffer solution. Buffer And Titration Practice Problems.
From www.youtube.com
Titration Practice Problems YouTube Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. A plot of ph versus volume of base added gives what is known as a titration curve. Must know the conjugate a/b concept to be. In order to understand buffers and buffer problems we must be proficient with the following.. Buffer And Titration Practice Problems.
From davida.davivienda.com
Titration Problems Worksheet Answers Printable Word Searches Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m. Explain how a buffer solution manages to stabilize the ph against the addition of. Buffer And Titration Practice Problems.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Buffer And Titration Practice Problems.
From www.studocu.com
217 buffer, titration problems CHEM 217 Studocu Buffer And Titration Practice Problems A plot of ph versus volume of base added gives what is known as a titration curve. In order to understand buffers and buffer problems we must be proficient with the following. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Must know the conjugate a/b concept to be.. Buffer And Titration Practice Problems.
From www.studocu.com
Titrationneutralization practice problems key Education Studocu Buffer And Titration Practice Problems Must know the conjugate a/b concept to be. A plot of ph versus volume of base added gives what is known as a titration curve. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. In order to understand buffers and buffer problems we must be proficient with the following.. Buffer And Titration Practice Problems.
From studylib.net
Titration Practice Worksheet Buffer And Titration Practice Problems A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The total molarity of acid and.. Buffer And Titration Practice Problems.
From studylib.net
acid base titration worksheet answer key Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Must know the conjugate a/b concept to be. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Consider the titration of. Buffer And Titration Practice Problems.
From www.scribd.com
Practice Problems Buffers PDF Ph Buffer Solution Buffer And Titration Practice Problems In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Must know the conjugate a/b concept to be. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The total molarity of. Buffer And Titration Practice Problems.
From www.scribd.com
Gen Chem II EX 4 Practice Problems Sp08 PDF Buffer Solution Titration Buffer And Titration Practice Problems The total molarity of acid and. A plot of ph versus volume of base added gives what is known as a titration curve. In the titration of 80.0 ml of 0.150 m ethylamine, c2h5nh2, with 0.100 m hcl, find the ph at each. Must know the conjugate a/b concept to be. In order to understand buffers and buffer problems we. Buffer And Titration Practice Problems.