Does Pressure Increase As Temperature Decreases . Pv = nrt, where p is pressure, v is volume, n is the number of moles of. In reality, most compression take place by reducing volume. These higher energy particles move faster,. When the temperature increases, the air molecules gain kinetic energy and move more quickly. As a result, they spread out, and. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. Yes, at constant density, the pressure increases as the temperature does: Yes, the relationship between pressure and temperature is described by the ideal gas law: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. For example, having water sealed at atmospheric pressure at. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two.
from www.teachoo.com
The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pv = nrt, where p is pressure, v is volume, n is the number of moles of. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. As a result, they spread out, and. These higher energy particles move faster,. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Yes, the relationship between pressure and temperature is described by the ideal gas law:
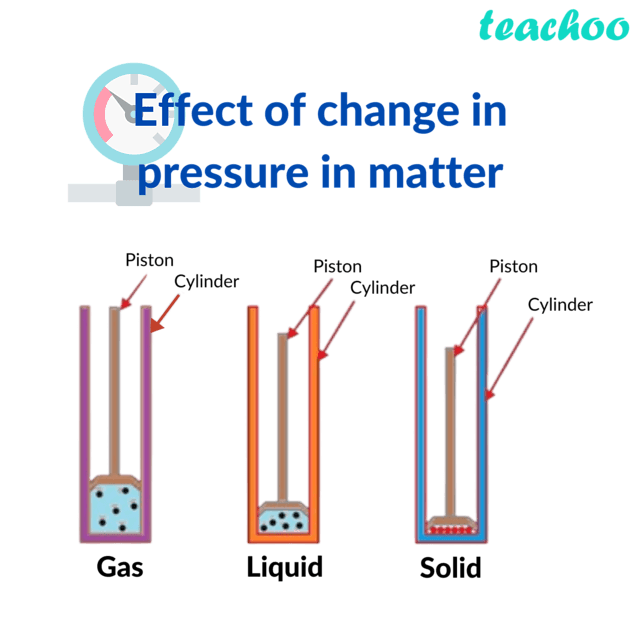
Changing Pressure to Change State of Matter Chemistry Teachoo
Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. For example, having water sealed at atmospheric pressure at. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Yes, the relationship between pressure and temperature is described by the ideal gas law: In reality, most compression take place by reducing volume. As a result, they spread out, and. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. These higher energy particles move faster,. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Increasing the temperature of a gas increases the pressure and the energy of the gas particles.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Pressure Increase As Temperature Decreases Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature increases, the air molecules gain kinetic energy and move more quickly. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Yes, the relationship. Does Pressure Increase As Temperature Decreases.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Does Pressure Increase As Temperature Decreases In reality, most compression take place by reducing volume. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). When the temperature increases, the air molecules gain kinetic energy and move more quickly. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. For example,. Does Pressure Increase As Temperature Decreases.
From www.chegg.com
Solved d) As the reaction occurs at constant temperature, Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. Yes, at constant density, the pressure increases as the temperature does: If you had a way to increase pressure with no volume change, then. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Pressure Increase As Temperature Decreases As a result, they spread out, and. These higher energy particles move faster,. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Yes, at constant density, the pressure increases as the temperature does: In reality, most compression take place. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID6013266 Does Pressure Increase As Temperature Decreases In reality, most compression take place by reducing volume. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. Yes, at constant density, the pressure increases as the temperature does: If you had a way to increase. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Chapter 13 Equilibrium PowerPoint Presentation, free download Does Pressure Increase As Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Yes, the relationship between pressure and temperature is described by the ideal gas law: As a result, they spread out, and. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. For. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT CLIMATE and BIOMES PowerPoint Presentation, free download ID Does Pressure Increase As Temperature Decreases In reality, most compression take place by reducing volume. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. When the temperature increases, the air molecules gain kinetic energy and move more quickly. As the temperature increases, the pressure increases showing that pressure. Does Pressure Increase As Temperature Decreases.
From slideplayer.com
Density. ppt download Does Pressure Increase As Temperature Decreases As a result, they spread out, and. Yes, at constant density, the pressure increases as the temperature does: Increasing the temperature of a gas increases the pressure and the energy of the gas particles. Yes, the relationship between pressure and temperature is described by the ideal gas law: When the temperature increases, the air molecules gain kinetic energy and move. Does Pressure Increase As Temperature Decreases.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Pressure Increase As Temperature Decreases In reality, most compression take place by reducing volume. Yes, at constant density, the pressure increases as the temperature does: When the temperature increases, the air molecules gain kinetic energy and move more quickly. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. The relationships among the volume of a gas and. Does Pressure Increase As Temperature Decreases.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Does Pressure Increase As Temperature Decreases These higher energy particles move faster,. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Yes, the relationship between pressure and temperature is described by the ideal gas law: As the temperature increases, the pressure increases showing that pressure. Does Pressure Increase As Temperature Decreases.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. For example, having water sealed at atmospheric pressure at. When the temperature of a particular system is increased, the molecules in the gas move. Does Pressure Increase As Temperature Decreases.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Does Pressure Increase As Temperature Decreases These higher energy particles move faster,. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Yes, the relationship between pressure and temperature is described by the ideal gas law: Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature of a. Does Pressure Increase As Temperature Decreases.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Pressure Increase As Temperature Decreases For example, having water sealed at atmospheric pressure at. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. These higher energy particles move faster,. Yes, at constant density, the pressure increases as the temperature does: If. Does Pressure Increase As Temperature Decreases.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does Pressure Increase As Temperature Decreases Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Yes, the relationship between pressure and temperature is described by the ideal gas law: For example, having. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. Yes, the relationship between pressure and temperature is described by the ideal gas law:. Does Pressure Increase As Temperature Decreases.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Does Pressure Increase As Temperature Decreases For example, having water sealed at atmospheric pressure at. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. As a result, they spread out, and. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. As the temperature increases, the. Does Pressure Increase As Temperature Decreases.
From slideplayer.com
If temperature decreases, what happens to pressure? ppt download Does Pressure Increase As Temperature Decreases In reality, most compression take place by reducing volume. Yes, the relationship between pressure and temperature is described by the ideal gas law: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a. Does Pressure Increase As Temperature Decreases.
From slideplayer.com
Behavior of Gases Gas Laws. ppt download Does Pressure Increase As Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. As a result, they spread. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID3552130 Does Pressure Increase As Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. For example, having water sealed at atmospheric pressure at. The relationships among the volume of a gas and its pressure, temperature,. Does Pressure Increase As Temperature Decreases.
From www.numerade.com
SOLVED 'The answer please will give brainliest Question 1 The general Does Pressure Increase As Temperature Decreases When the temperature increases, the air molecules gain kinetic energy and move more quickly. In reality, most compression take place by reducing volume. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. When the temperature of. Does Pressure Increase As Temperature Decreases.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Pressure Increase As Temperature Decreases Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. When the temperature increases, the air molecules gain kinetic energy and move more. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does Pressure Increase As Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Yes, the relationship between pressure and temperature is described by the ideal gas law: For example, having water sealed at atmospheric pressure at. When. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Unit 6 Atmospheric Stability PowerPoint Presentation, free Does Pressure Increase As Temperature Decreases Yes, at constant density, the pressure increases as the temperature does: Pv = nrt, where p is pressure, v is volume, n is the number of moles of. In reality, most compression take place by reducing volume. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As a result, they. Does Pressure Increase As Temperature Decreases.
From www.zigya.com
With the help of suitable diagram, describe the variation in (i Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). For example, having water sealed at atmospheric pressure at. In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Yes,. Does Pressure Increase As Temperature Decreases.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Does Pressure Increase As Temperature Decreases As a result, they spread out, and. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. Pv = nrt, where p is pressure, v is volume, n is the number. Does Pressure Increase As Temperature Decreases.
From www.pinterest.com
Boyles Law States that pressure increases when volume decreases, and Does Pressure Increase As Temperature Decreases Yes, the relationship between pressure and temperature is described by the ideal gas law: For example, having water sealed at atmospheric pressure at. Yes, at constant density, the pressure increases as the temperature does: As a result, they spread out, and. Pv = nrt, where p is pressure, v is volume, n is the number of moles of. As the. Does Pressure Increase As Temperature Decreases.
From slideplayer.com
Observations, Measurements, and Change ppt download Does Pressure Increase As Temperature Decreases As a result, they spread out, and. Increasing the temperature of a gas increases the pressure and the energy of the gas particles. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. When the temperature of a particular system is increased, the molecules in the gas move. Does Pressure Increase As Temperature Decreases.
From brainly.ph
TEMPERATURE INCREASES/PRESSURE DECREASESAсDEFBTEMPERATURE DECREASES Does Pressure Increase As Temperature Decreases Yes, at constant density, the pressure increases as the temperature does: When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. When the temperature increases, the air molecules gain kinetic energy and move more quickly. Yes, the relationship between pressure and temperature is. Does Pressure Increase As Temperature Decreases.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Pressure Increase As Temperature Decreases When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. For example, having water sealed at atmospheric pressure at. These higher energy particles move faster,. When the temperature increases, the air molecules gain kinetic energy and move more quickly. The relationships among the. Does Pressure Increase As Temperature Decreases.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Increase As Temperature Decreases Yes, at constant density, the pressure increases as the temperature does: As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. Yes, the relationship between pressure and temperature is described by the ideal gas law: Pv = nrt, where p is pressure, v is volume, n is the number of moles of. If. Does Pressure Increase As Temperature Decreases.
From www.youtube.com
Why temperature decreases with altitude?why_temperature_decreases_with Does Pressure Increase As Temperature Decreases Pv = nrt, where p is pressure, v is volume, n is the number of moles of. Yes, at constant density, the pressure increases as the temperature does: When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. For example, having water sealed. Does Pressure Increase As Temperature Decreases.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase As Temperature Decreases For example, having water sealed at atmospheric pressure at. When the temperature of a particular system is increased, the molecules in the gas move faster, exerting a greater pressure on the wall of the gas container. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. As a result, they spread out, and.. Does Pressure Increase As Temperature Decreases.
From courses.lumenlearning.com
Phase Changes Physics Does Pressure Increase As Temperature Decreases As a result, they spread out, and. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. For example, having water sealed at atmospheric pressure at. Yes, the relationship between pressure and temperature is described by the ideal gas law: When the temperature increases, the air molecules gain. Does Pressure Increase As Temperature Decreases.
From www.slideshare.net
Air pressure Does Pressure Increase As Temperature Decreases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pv = nrt, where p is pressure, v is volume, n is the number of moles of. These higher energy particles move faster,. As the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. For. Does Pressure Increase As Temperature Decreases.
From slideplayer.com
What is Density? is the amount of matter in a specific volume. ppt Does Pressure Increase As Temperature Decreases Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pv = nrt, where p is pressure, v is volume, n is the number of moles of. These higher energy particles move faster,. Yes, the relationship between pressure and temperature. Does Pressure Increase As Temperature Decreases.