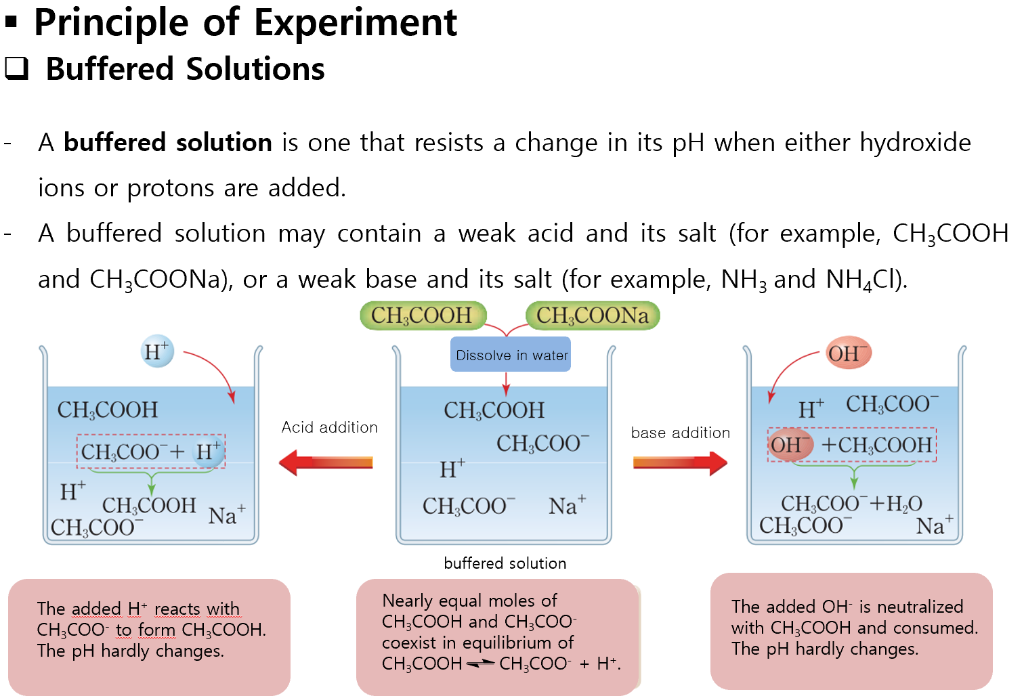
What Is Considered A Buffer Solution . A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. Buffer solutions resist a change in ph. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Primarily buffer solutions are of two types: An acidic buffer solution is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.
from allyson-has-mccoy.blogspot.com
Buffer solutions resist a change in ph. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Primarily buffer solutions are of two types: A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An acidic buffer solution is. It is able to neutralize small amounts of added acid or base, thus.
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
What Is Considered A Buffer Solution A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Primarily buffer solutions are of two types: A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. An acidic buffer solution is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffer solutions resist a change in ph.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is Considered A Buffer Solution It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Primarily buffer solutions are of two types: Buffer solutions resist a change in ph. An acidic buffer solution is. A buffer solution is one which. What Is Considered A Buffer Solution.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog What Is Considered A Buffer Solution Primarily buffer solutions are of two types: Buffer solutions resist a change in ph. An acidic buffer solution is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small amounts of added acid or base,. What Is Considered A Buffer Solution.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is Considered A Buffer Solution A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is Considered A Buffer Solution A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change in ph. Primarily buffer solutions are of two types: It is able to neutralize small amounts of added acid or base, thus. A buffer solution prepared with large quantities of a weak. What Is Considered A Buffer Solution.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer What Is Considered A Buffer Solution Buffer solutions resist a change in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A buffer solution is one which resists changes in ph when. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Considered A Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Primarily buffer solutions are of two types: A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An. What Is Considered A Buffer Solution.
From www.pdfprof.com
PDF examples of buffer solutions PDF Télécharger Download What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution. What Is Considered A Buffer Solution.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy What Is Considered A Buffer Solution It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer. What Is Considered A Buffer Solution.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download What Is Considered A Buffer Solution Buffer solutions resist a change in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A buffer solution is one which resists changes in ph when. What Is Considered A Buffer Solution.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is Considered A Buffer Solution A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A buffer is a solution that maintains the stability of a system’s ph level. What Is Considered A Buffer Solution.
From fr.slideshare.net
Buffer solution What Is Considered A Buffer Solution A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A mixture. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A mixture of a weak. What Is Considered A Buffer Solution.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is Considered A Buffer Solution Primarily buffer solutions are of two types: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An acidic buffer solution is. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak. What Is Considered A Buffer Solution.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. An acidic buffer solution is. A buffer is a solution that maintains. What Is Considered A Buffer Solution.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Is Considered A Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is Considered A Buffer Solution.
From www.toppr.com
Buffer Solutions Definition, Types, Preparation, Examples and Videos What Is Considered A Buffer Solution Primarily buffer solutions are of two types: A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a. What Is Considered A Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is Considered A Buffer Solution Primarily buffer solutions are of two types: A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base,. What Is Considered A Buffer Solution.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is Considered A Buffer Solution Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. An acidic buffer solution is. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. What Is Considered A Buffer Solution.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation What Is Considered A Buffer Solution An acidic buffer solution is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution prepared with large quantities. What Is Considered A Buffer Solution.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution What Is Considered A Buffer Solution Buffer solutions resist a change in ph. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A. What Is Considered A Buffer Solution.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is Considered A Buffer Solution It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Considered A Buffer Solution A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution prepared with large quantities of a weak acid, and its salt with. What Is Considered A Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is Considered A Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. An acidic buffer solution is. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. It is able. What Is Considered A Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution prepared with large quantities of a weak acid, and. What Is Considered A Buffer Solution.
From www.youtube.com
Buffer Solutions YouTube What Is Considered A Buffer Solution Primarily buffer solutions are of two types: It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is Considered A Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is Considered A Buffer Solution A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID4273107 What Is Considered A Buffer Solution A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer. What Is Considered A Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Is Considered A Buffer Solution A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Considered A Buffer Solution An acidic buffer solution is. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Primarily buffer solutions are of two types: A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. It is. What Is Considered A Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Is Considered A Buffer Solution Primarily buffer solutions are of two types: A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A mixture of a weak acid and. What Is Considered A Buffer Solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is Considered A Buffer Solution A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. Primarily buffer solutions are of two types: Buffer solutions resist a change in ph. A buffer solution is one which resists changes in ph when. What Is Considered A Buffer Solution.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is Considered A Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution prepared with large quantities of a weak acid, and its salt with a strong base, is known as an acid. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and. What Is Considered A Buffer Solution.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is Considered A Buffer Solution A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An acidic buffer solution is. Buffer solutions resist a change in ph. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a. What Is Considered A Buffer Solution.
From golifescience.com
Buffer Solution definition, 4 Types and Basic Calculations What Is Considered A Buffer Solution A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small amounts of added. What Is Considered A Buffer Solution.