Catalyst Reaction Function . Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance. What are catalysts, and how do they work in terms altering the parameters of a reaction? In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from www.dreamstime.com
A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption.
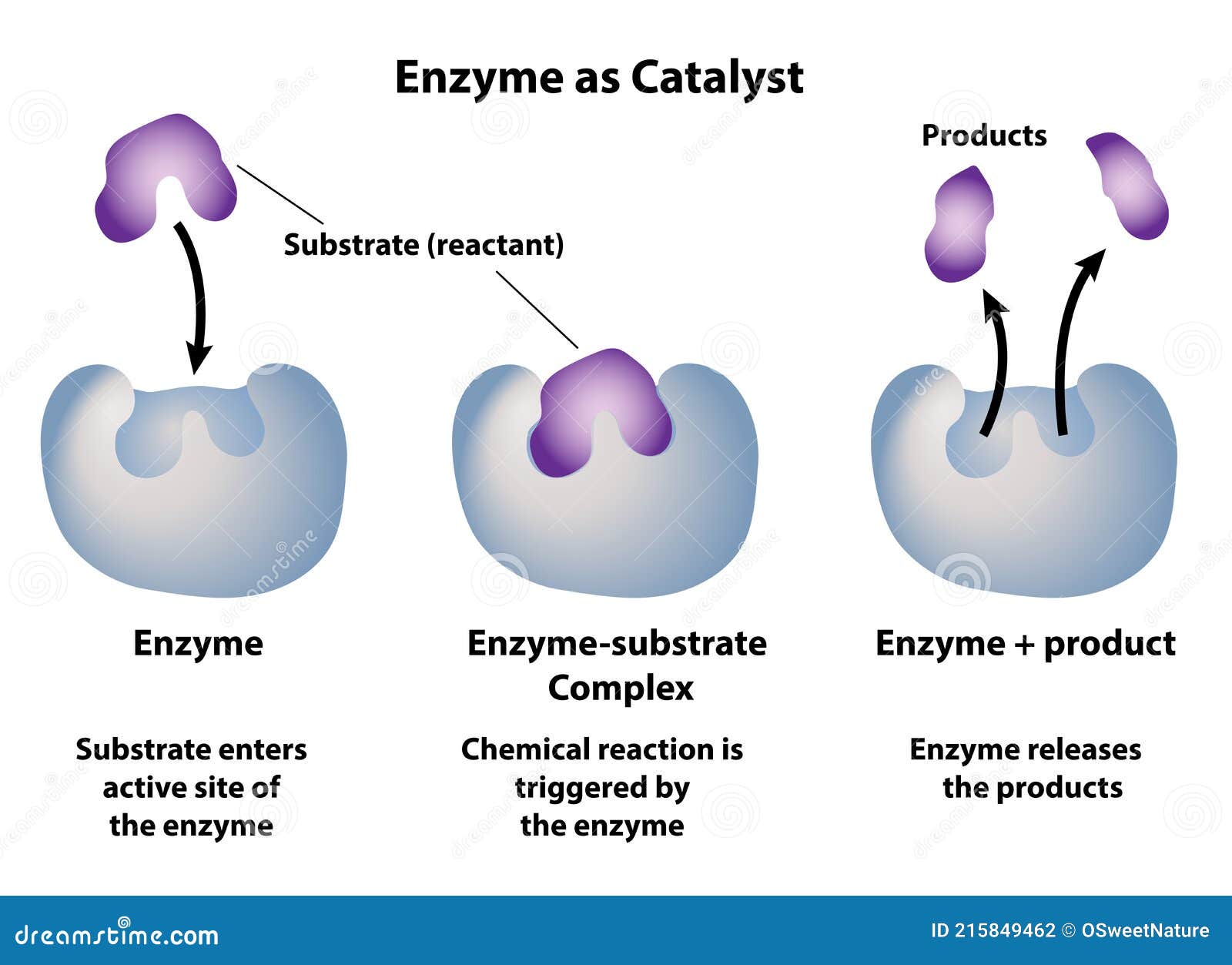
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of
Catalyst Reaction Function Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance. List examples of catalysis in natural. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Reaction Function Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. What are catalysts, and how do they work in terms altering the parameters of a reaction? Explain the. Catalyst Reaction Function.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Reaction Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.. Catalyst Reaction Function.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Function Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the. Catalyst Reaction Function.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Reaction Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. What are catalysts, and how do they work in terms altering. Catalyst Reaction Function.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Reaction Function List examples of catalysis in natural. A catalyst is a chemical substance. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. In heterogeneous catalysis, catalysts provide a surface to. Catalyst Reaction Function.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Function List examples of catalysis in natural. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction. Catalyst Reaction Function.
From www.slideshare.net
Catalysis Catalyst Reaction Function Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. A catalyst binds to a reactant and it increases the number. Catalyst Reaction Function.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Reaction Function A catalyst is a chemical substance. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that. Catalyst Reaction Function.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. List examples of catalysis in natural. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams.. Catalyst Reaction Function.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Reaction Function A catalyst is a chemical substance. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required. Catalyst Reaction Function.
From learningcampusstall.z21.web.core.windows.net
Enzyme Catalysed Reaction Graph Catalyst Reaction Function What are catalysts, and how do they work in terms altering the parameters of a reaction? Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance. List examples of catalysis in natural. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules,. Catalyst Reaction Function.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Reaction Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Explain the function of a. Catalyst Reaction Function.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Reaction Function In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst binds to a reactant and. Catalyst Reaction Function.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Reaction Function In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms. Catalyst Reaction Function.
From ch302.cm.utexas.edu
chem1 Catalyst Reaction Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst binds. Catalyst Reaction Function.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. List examples of catalysis in natural. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction. Catalyst Reaction Function.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Reaction Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance that affects the rate of a chemical reaction by. Catalyst Reaction Function.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and. Catalyst Reaction Function.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to proceed via a pathway that has a. Catalyst Reaction Function.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Reaction Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Explain the function of a catalyst in terms of reaction mechanisms. Catalyst Reaction Function.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering. Catalyst Reaction Function.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Catalyst Reaction Function Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is a chemical substance. Catalysts allow a reaction to. Catalyst Reaction Function.
From www.youtube.com
Identifying function of catalyst in a reaction YouTube Catalyst Reaction Function Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance. What are catalysts, and how do they work in terms altering the parameters of a reaction? In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A catalyst is a chemical substance that. Catalyst Reaction Function.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of. Catalyst Reaction Function.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural.. Catalyst Reaction Function.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Reaction Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. List examples of catalysis in natural. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalyst Reaction Function.
From biologyease.com
Mechanism of Enzyme Catalysis Biology Ease Catalyst Reaction Function What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance. A catalyst binds to a reactant and it increases the number of collision. Catalyst Reaction Function.
From large.stanford.edu
Catalysts in 21st Century Energy Catalyst Reaction Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts allow a reaction to proceed via a pathway that has. Catalyst Reaction Function.
From www.researchgate.net
Typical NOSCO catalyst identification mappings as functions of Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the. Catalyst Reaction Function.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Reaction Function A catalyst is a chemical substance. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. List examples of catalysis in natural. A catalyst binds to a reactant and it increases the number. Catalyst Reaction Function.
From zymvol.com
All you need to know about enzymes Zymvol Catalyst Reaction Function In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in. Catalyst Reaction Function.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Reaction Function A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is. Catalyst Reaction Function.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Reaction Function In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Explain the function of a catalyst in terms of reaction mechanisms and. Catalyst Reaction Function.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst Reaction Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. What are catalysts, and how do they work in terms altering the parameters of a reaction? Explain the function of a catalyst in terms of reaction. Catalyst Reaction Function.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalyst Reaction Function List examples of catalysis in natural. A catalyst is a chemical substance. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a. Catalyst Reaction Function.