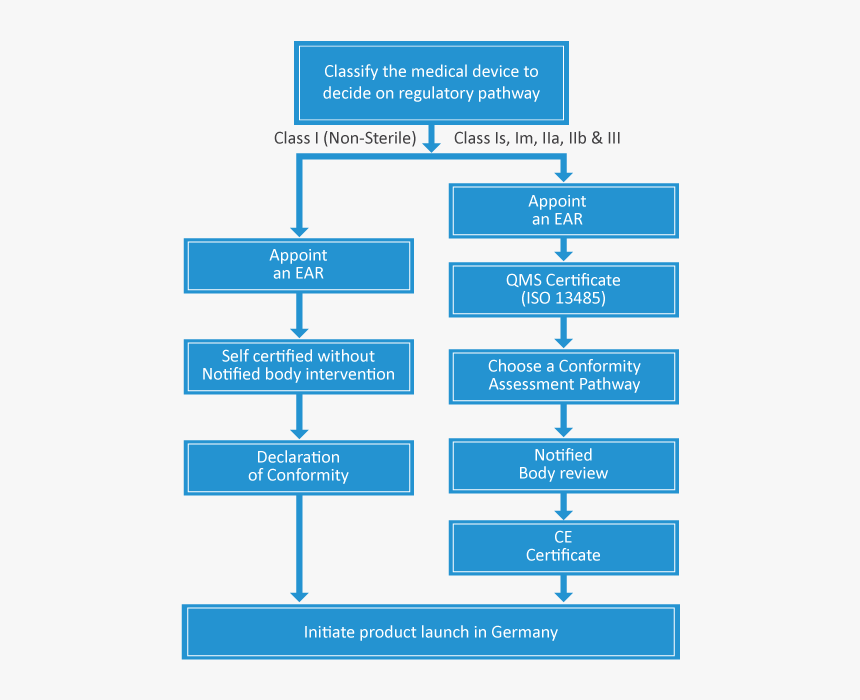
Medical Device Registration Germany . Some of the member states and those participating in the single market require additional registration. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Class i being the lowest risk while class iii being the highest risk. Applying the ce mark allows your devices to easily be imported and sold throughout europe. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional requirements, our experts. I, iia, iib, and iii. Operon strategist offers a positive and efficient approach to medical device registration in germany.
from www.pngitem.com
The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional requirements, our experts. Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Applying the ce mark allows your devices to easily be imported and sold throughout europe. Some of the member states and those participating in the single market require additional registration. Operon strategist offers a positive and efficient approach to medical device registration in germany. I, iia, iib, and iii.
Germany Market Entry Strategy Medical Device Registration Process In
Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. I, iia, iib, and iii. Operon strategist offers a positive and efficient approach to medical device registration in germany. Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Some of the member states and those participating in the single market require additional registration.
From www.rizmona.com
Medical Device Registration MOH Product Classification Riz & Mona Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. Some of the member states and those participating in the single market require additional registration. Operon strategist offers a positive and efficient approach to medical device registration in germany. I, iia, iib, and iii. The central. Medical Device Registration Germany.
From vyomusconsulting.com
MedicalDevice & IVD Registration Process Overview Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. From ce marking to navigating additional requirements, our experts. Operon strategist offers a positive and efficient approach to medical device registration in germany.. Medical Device Registration Germany.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Operon strategist offers a positive and efficient approach to medical device registration in germany. Applying the ce mark allows your devices to easily be imported. Medical Device Registration Germany.
From cmsmedtech.com
medical device registration in Europe Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Operon strategist offers a positive and efficient approach to medical device registration in germany. I, iia, iib, and iii.. Medical Device Registration Germany.
From www.reghelps.com
Medical Device Registration & Device Listing Best FDA Specialist Medical Device Registration Germany The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Applying the ce mark allows your devices to easily be imported and. Medical Device Registration Germany.
From www.chameleon-pharma.com
MD Registration in Russia & EAEU Chameleon Pharma Consulting Medical Device Registration Germany The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. From ce marking to navigating additional requirements, our experts. Some of the member states and those participating in the single market require additional registration. Operon strategist offers a positive and efficient approach to medical device registration in germany.. Medical Device Registration Germany.
From www.youtube.com
European Medical Device Registration Chapter 1 Overview YouTube Medical Device Registration Germany I, iia, iib, and iii. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Some of the member states and those participating in the single market require additional registration. Applying the ce mark allows your devices to easily be imported and sold throughout. Medical Device Registration Germany.
From www.freyrsolutions.com
Medical Devices and CE Marking Process under the EU MDR Freyr Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. I, iia, iib, and iii. Some of the member states and those participating in the single market require additional registration. Operon strategist offers a positive. Medical Device Registration Germany.
From gxpcellators.com
FDA Medical Device Registration Process Guide Medical Device Registration Germany The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Class i being the lowest risk while class iii being the highest risk. Operon strategist offers a positive and efficient approach to medical device registration in germany. The bfarm main tasks include licensing, improving the safety of medicinal. Medical Device Registration Germany.
From casusconsulting.com
2024 Steps to Enter the EU Market. Free Flowchart. Casus Consulting Medical Device Registration Germany I, iia, iib, and iii. Applying the ce mark allows your devices to easily be imported and sold throughout europe. Operon strategist offers a positive and efficient approach to medical device registration in germany. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. The bfarm main tasks. Medical Device Registration Germany.
From operonstrategist.com
Medical Device Regulations in Germany Operon Strategist Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. From ce marking to navigating additional requirements, our experts. I, iia, iib, and iii. Some of the member states and those participating in the single. Medical Device Registration Germany.
From chinameddevice.com
NMPA Medical Device Registration & Clinical Trial Process Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. I,. Medical Device Registration Germany.
From www.cirs-group.com
[Important] How to Check the Registration Information of Antiepidemic Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Some of the member states and those participating in the single market require additional registration. From ce marking to navigating additional requirements, our experts.. Medical Device Registration Germany.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. I, iia, iib, and iii. From ce marking to navigating additional requirements, our experts. Applying the ce mark allows your devices to easily be imported and sold throughout europe. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks. Medical Device Registration Germany.
From www.freyrsolutions.com
Medical Device Registration Process in China Freyr Global Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. Class i being the lowest risk while class iii being the highest risk. Applying the ce mark allows your devices to easily be imported and sold throughout europe. I, iia, iib, and iii. Operon strategist offers a positive and efficient approach to medical device registration. Medical Device Registration Germany.
From germanmarketaccesssimplified.com
Reimbursement of medical devices in Germany Medical Device Registration Germany The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Class i being the lowest risk while class iii being the highest risk. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic. Medical Device Registration Germany.
From coat-x.com
COATX gets its ISO13485 Medical Device certificate COATX Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. From ce marking to navigating additional requirements, our experts. Class i being the lowest risk while class iii being the highest risk. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal. Medical Device Registration Germany.
From www.regdesk.co
HSA Guidance on Medical Device Product Registration Class C and D Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. Class i being the lowest risk while class iii being the highest risk. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional. Medical Device Registration Germany.
From patientguard.com
Medical Device and IVD Registration Medical Device Registration Germany From ce marking to navigating additional requirements, our experts. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Operon strategist offers a positive and efficient approach to medical device registration in germany. Class i being the lowest risk while class iii being the highest risk. Applying the. Medical Device Registration Germany.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Registration Germany The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional requirements, our experts. Applying the ce mark allows your devices to easily be imported and sold throughout europe. Some of the member states and those participating in the. Medical Device Registration Germany.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. I, iia, iib, and iii. Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. The central authority of the laender for health protection with regard to medicinal products and medical. Medical Device Registration Germany.
From kc-prof.ru
Registration Certificate for a Medical Device KCPROF Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. I, iia, iib, and iii. From ce marking to navigating additional requirements, our experts. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. The central authority of the. Medical Device Registration Germany.
From cmsmedtech.com
Medical Device Registration in EAEU Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. Class i being the lowest risk while class iii being the highest risk. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Some of the member states and those participating in the single. Medical Device Registration Germany.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. I, iia, iib, and iii. Some of the member states and those participating in the single market require additional registration. Class i being the lowest risk while class iii being the highest risk. The bfarm main tasks include licensing, improving the safety of medicinal products,. Medical Device Registration Germany.
From www.regdesk.co
Medical Device Regulations in Germany RegDesk Professional Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. Some of the member states and those participating in the single market require additional registration. Class i being the lowest risk while class iii being the highest risk. I, iia, iib, and iii. The central authority of the laender for health protection with regard to. Medical Device Registration Germany.
From diaztradelaw.com
Tips on FDA's Medical Device Registration Process Customs Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Some of the member states and those participating in the single market require additional registration. I, iia, iib, and iii.. Medical Device Registration Germany.
From www.scribd.com
EC Certificate PDF Medical Device Certification Medical Device Registration Germany From ce marking to navigating additional requirements, our experts. Class i being the lowest risk while class iii being the highest risk. I, iia, iib, and iii. Operon strategist offers a positive and efficient approach to medical device registration in germany. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical. Medical Device Registration Germany.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Medical Device Registration Germany Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Operon strategist offers a positive and efficient approach to medical. Medical Device Registration Germany.
From scientistssanctuary.com
eBOOK How to Achieve European Medical Device Registration and Apply a Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. Applying the ce mark allows your devices to easily be imported and sold throughout europe. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. I, iia, iib, and iii. From. Medical Device Registration Germany.
From www.pngitem.com
Germany Market Entry Strategy Medical Device Registration Process In Medical Device Registration Germany Class i being the lowest risk while class iii being the highest risk. Operon strategist offers a positive and efficient approach to medical device registration in germany. Applying the ce mark allows your devices to easily be imported and sold throughout europe. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of. Medical Device Registration Germany.
From www.techsollifesciences.com
Medical Device Registration Regulatory Requirements Medical Device Registration Germany I, iia, iib, and iii. The central authority of the laender for health protection with regard to medicinal products and medical devices (zlg) is the german authority. Applying the ce mark allows your devices to easily be imported and sold throughout europe. From ce marking to navigating additional requirements, our experts. Class i being the lowest risk while class iii. Medical Device Registration Germany.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. Class i being the lowest risk while class iii being the highest risk. Operon strategist offers a positive and efficient approach to medical device registration in germany. From ce marking to navigating additional requirements, our experts. The bfarm main tasks include licensing, improving the safety. Medical Device Registration Germany.
From www.kolabtree.com
Bereiten Sie Ihr Medizinprodukt auf die EUMDR vor 8 bewährte Ressourcen Medical Device Registration Germany The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. Operon strategist offers a positive and efficient approach to medical device registration in germany. Applying the ce mark allows your devices to easily be imported and sold throughout europe. I, iia, iib, and iii.. Medical Device Registration Germany.
From medicaldevices.freyrsolutions.com
US FDA Medical Device Registration, US FDA Agent Medical Device Registration Germany Some of the member states and those participating in the single market require additional registration. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional requirements, our experts. The central authority of the laender for health protection with. Medical Device Registration Germany.
From operonstrategist.com
Guide On Medical Device Registration In 9 Different Countries Medical Device Registration Germany Operon strategist offers a positive and efficient approach to medical device registration in germany. The bfarm main tasks include licensing, improving the safety of medicinal products, detecting and evaluating the risks of medical devices, and monitoring the legal traffic in. From ce marking to navigating additional requirements, our experts. Class i being the lowest risk while class iii being the. Medical Device Registration Germany.