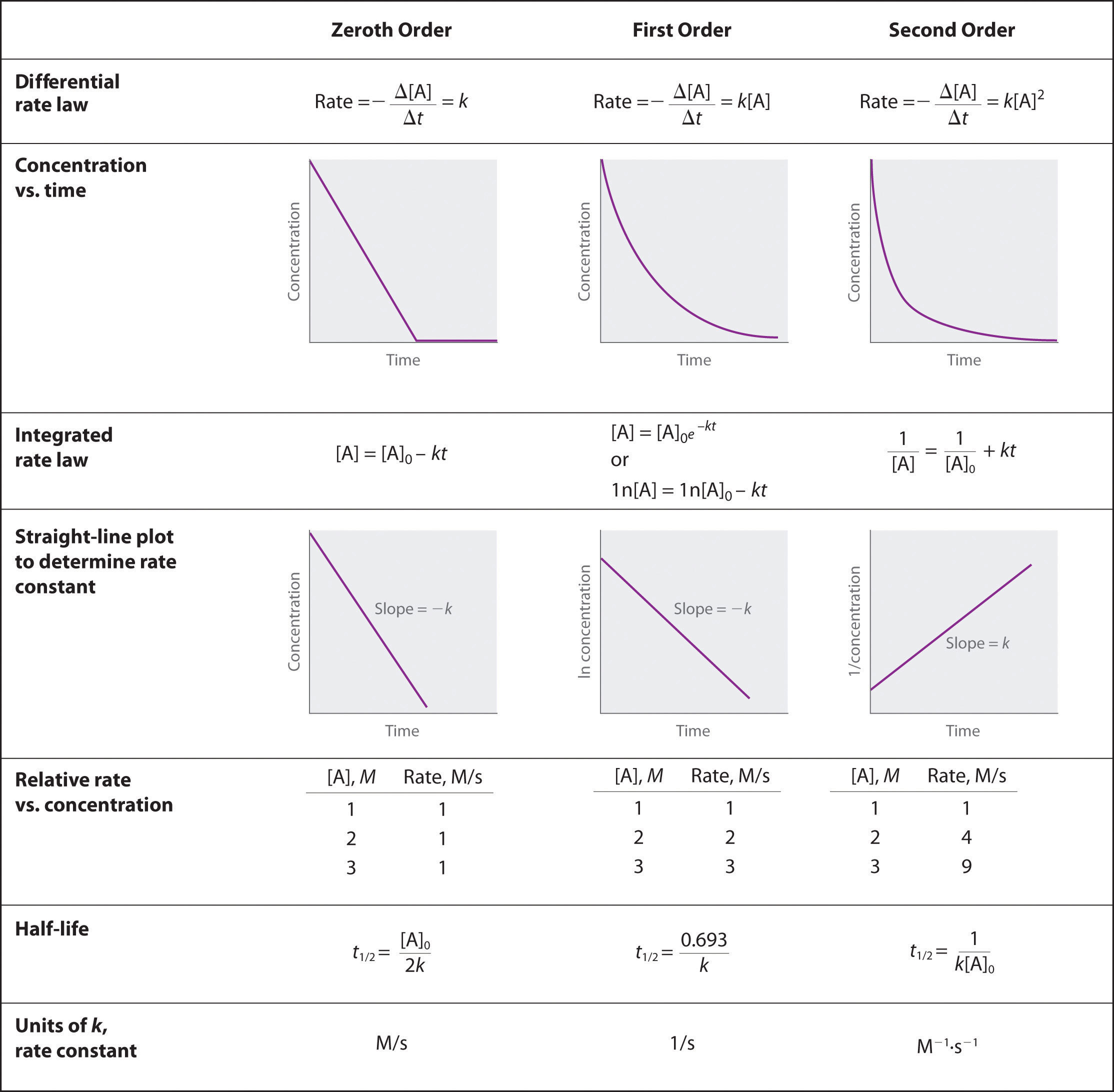
Rate Constant Calculation From Graph . This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Use rate laws to calculate. By the end of this section, you will be able to: The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Rate constants and the arrhenius equation. Once the graph shows a straight line, the rate constant is found from the slope of the line. Explain the form and function of a rate law. Using the data for t = 0 s.
from 2012books.lardbucket.org
Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Use rate laws to calculate. Explain the form and function of a rate law. Using the data for t = 0 s. By the end of this section, you will be able to: The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Once the graph shows a straight line, the rate constant is found from the slope of the line.
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders
Rate Constant Calculation From Graph Rate constants and the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Use rate laws to calculate. By the end of this section, you will be able to: Rate constants and the arrhenius equation. Once the graph shows a straight line, the rate constant is found from the slope of the line. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Using the data for t = 0 s. Explain the form and function of a rate law.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Rate constants and the arrhenius equation. Using the data for t = 0 s. Explain the form and function of. Rate Constant Calculation From Graph.
From general.chemistrysteps.com
Determining Reaction Order Using Graphs Chemistry Steps Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Rate constants and the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Using the data for t = 0 s. Explain the form and. Rate Constant Calculation From Graph.
From www.youtube.com
Rate equation and the units of the rate constant YouTube Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Explain the form and function of a rate law. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Use. Rate Constant Calculation From Graph.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant Calculation From Graph Once the graph shows a straight line, the rate constant is found from the slope of the line. Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Use rate laws to calculate. Explain the form and function of a rate law. Plot. Rate Constant Calculation From Graph.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Using the data for t = 0 s. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Use rate laws to calculate. Plot a graph. Rate Constant Calculation From Graph.
From www.youtube.com
How to calculate the order of a reaction and the rate constant YouTube Rate Constant Calculation From Graph Rate constants and the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Once the graph shows a straight line, the rate constant is found from the slope of the line. Use rate laws to calculate. Explain the form and function of a rate. Rate Constant Calculation From Graph.
From www.youtube.com
First Order Elimination Rate Constant and Halflife A closer look Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Rate constants and the arrhenius equation. Using the data for t = 0 s. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration. Rate Constant Calculation From Graph.
From chart-studio.plotly.com
Rate constant in function of temperature scatter chart made by Ayoub Rate Constant Calculation From Graph Once the graph shows a straight line, the rate constant is found from the slope of the line. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Explain the form and function of a rate law. By the end of this section, you will be able to: Plot. Rate Constant Calculation From Graph.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Once the graph shows a straight line, the rate constant is found from the slope of the line. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a. Rate Constant Calculation From Graph.
From www.youtube.com
Determining Rate Laws from Experimental Data YouTube Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t. Rate Constant Calculation From Graph.
From morioh.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Explain the form and function of a rate law. Once the graph shows a straight line, the rate constant is found from the slope of the line. This page looks at the way that rate constants vary. Rate Constant Calculation From Graph.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant Calculation From Graph Rate constants and the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Using the data for t = 0 s. Once the graph shows a straight line, the rate constant is found from the slope of the line. Explain the form and function. Rate Constant Calculation From Graph.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Using the data for t = 0 s. Once the graph shows a straight line, the rate constant is found from the slope of the line. Explain the form and function of a rate law. The arrhenius plot is. Rate Constant Calculation From Graph.
From www.researchgate.net
Graphs used to back calculate the rate constants for the recovery of Rate Constant Calculation From Graph Using the data for t = 0 s. Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Plot a graph of the concentration. Rate Constant Calculation From Graph.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Once the graph shows a straight line, the rate constant is found. Rate Constant Calculation From Graph.
From www.doubtnut.com
Rate constant vs temperature graph looks like If the activation energ Rate Constant Calculation From Graph Use rate laws to calculate. Using the data for t = 0 s. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. By the end. Rate Constant Calculation From Graph.
From saylordotorg.github.io
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant Calculation From Graph Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. By the end of this section,. Rate Constant Calculation From Graph.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and Rate Constant Calculation From Graph Rate constants and the arrhenius equation. Once the graph shows a straight line, the rate constant is found from the slope of the line. By the end of this section, you will be able to: Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Using the. Rate Constant Calculation From Graph.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6810274 Rate Constant Calculation From Graph Explain the form and function of a rate law. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. This. Rate Constant Calculation From Graph.
From www.tessshebaylo.com
Derive Rate Constant Equation For Second Order Reaction Tessshebaylo Rate Constant Calculation From Graph Explain the form and function of a rate law. Rate constants and the arrhenius equation. Using the data for t = 0 s. Once the graph shows a straight line, the rate constant is found from the slope of the line. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t.. Rate Constant Calculation From Graph.
From demonstrations.wolfram.com
Rate Constant Calculation from Four Temperature and Moisture Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Explain the form and function of a rate law. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Use rate laws to calculate. Plot a. Rate Constant Calculation From Graph.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant Calculation From Graph Use rate laws to calculate. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Using the data for t. Rate Constant Calculation From Graph.
From www.slideserve.com
PPT METHODS OF DETERMINING ABSORPTION RATE CONSTANT PowerPoint Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Using the data for t = 0 s. Plot a graph of the concentration versus t, ln concentration versus t,. Rate Constant Calculation From Graph.
From www.chegg.com
Solved Calculate the value of the true rate constant, k, Rate Constant Calculation From Graph Use rate laws to calculate. Explain the form and function of a rate law. Once the graph shows a straight line, the rate constant is found from the slope of the line. By the end of this section, you will be able to: The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse. Rate Constant Calculation From Graph.
From www.youtube.com
Units of rate constant YouTube Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Explain the form and function of a rate law. Use rate laws to calculate. By the end of this section, you will be able to: Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration. Rate Constant Calculation From Graph.
From www.showme.com
Math 7 Constant Rate of Change (graph) Math, 7th grade math Rate Constant Calculation From Graph Explain the form and function of a rate law. By the end of this section, you will be able to: Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. The arrhenius plot is obtained by plotting the logarithm of the rate constant,. Rate Constant Calculation From Graph.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Using the data for t = 0 s. Use rate laws to calculate. Plot a graph of the concentration versus. Rate Constant Calculation From Graph.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Using the data for t = 0 s. By the end of this section, you will be able to: Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law. Rate Constant Calculation From Graph.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant Calculation From Graph Rate constants and the arrhenius equation. By the end of this section, you will be able to: Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the. Rate Constant Calculation From Graph.
From www.youtube.com
Rate of Change from a Graph YouTube Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. By the end of this section, you will be able to: Using the data for t = 0 s. Rate constants and the arrhenius equation. Use rate laws to calculate. Once the graph shows a straight line,. Rate Constant Calculation From Graph.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Rate Constant Calculation From Graph Once the graph shows a straight line, the rate constant is found from the slope of the line. By the end of this section, you will be able to: Rate constants and the arrhenius equation. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Use rate laws to calculate. Plot. Rate Constant Calculation From Graph.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant Calculation From Graph Use rate laws to calculate. The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. Explain the form and function of a rate law. Rate constants and the arrhenius equation. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation.. Rate Constant Calculation From Graph.
From www.youtube.com
KAC25.6 Rates II Rate Constants from Rateconcentration Plots YouTube Rate Constant Calculation From Graph This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and. Once the graph shows a straight line, the rate constant is found from the slope of. Rate Constant Calculation From Graph.
From www.youtube.com
Determining the Rate Law Using Initial Rates Data Example (Pt 1 of 3 Rate Constant Calculation From Graph The arrhenius plot is obtained by plotting the logarithm of the rate constant, k, versus the inverse temperature, 1/t. This page looks at the way that rate constants vary with temperature and activation energy as shown by the arrhenius equation. Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the. Rate Constant Calculation From Graph.
From chart-studio.plotly.com
Calculation of PseudoFirstOrder Rate Constant scatter chart made by Rate Constant Calculation From Graph Plot a graph of the concentration versus t, ln concentration versus t, and 1/concentration versus t and then determine the rate law and calculate the rate constant. Use rate laws to calculate. By the end of this section, you will be able to: Explain the form and function of a rate law. Once the graph shows a straight line, the. Rate Constant Calculation From Graph.