Chloride Ion The Charge . chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Since the neutral halogen atom. Chloride salts such as sodium chloride are often. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the chloride ion has a single, negative charge. 93 rows ionic charge: the chlorine ion is an anion (negatively charged ion) with the charge cl −. Example \(\pageindex{1}\) for each ion,.
from www.numerade.com
Chloride salts such as sodium chloride are often. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: the chlorine ion is an anion (negatively charged ion) with the charge cl −. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the chloride ion has a single, negative charge. 93 rows ionic charge: Example \(\pageindex{1}\) for each ion,. Since the neutral halogen atom. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
When chlorine gains an electron to a chloride ion with a 21
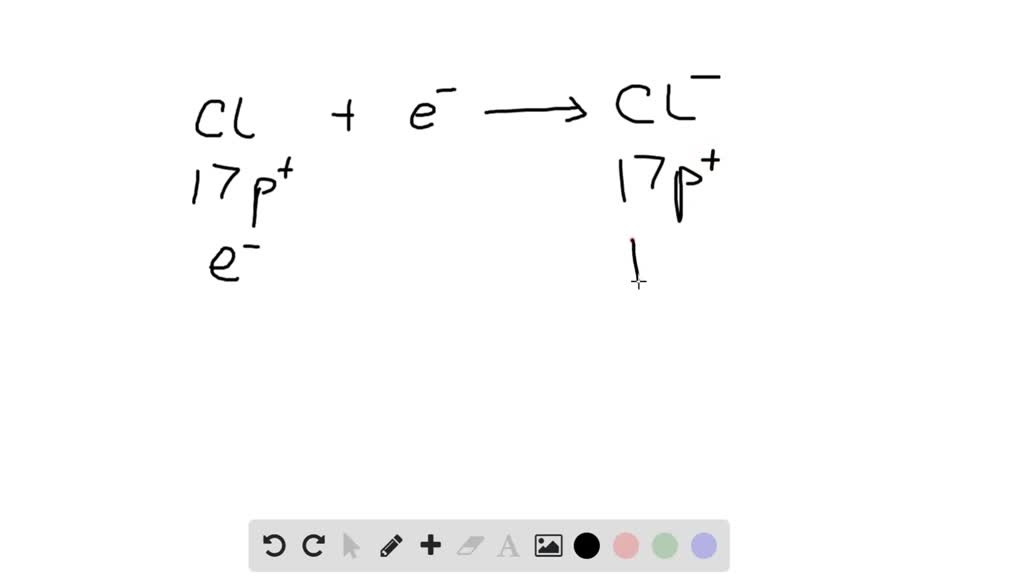
Chloride Ion The Charge the chloride ion has a single, negative charge. Example \(\pageindex{1}\) for each ion,. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. the chlorine ion is an anion (negatively charged ion) with the charge cl −. the chloride ion has a single, negative charge. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chloride salts such as sodium chloride are often. 93 rows ionic charge: Since the neutral halogen atom. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. Example \(\pageindex{1}\) for each ion,. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chloride salts such as sodium chloride are often. for the ionic compound between mg 2 + ions and cl. Chloride Ion The Charge.
From exowfmlsz.blob.core.windows.net
Chlorine Ion Charge Formula at Angela Orlando blog Chloride Ion The Charge for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Chloride salts such as sodium chloride are often. the chlorine ion is an anion (negatively charged ion) with the charge cl −. Example \(\pageindex{1}\) for each ion,. when forming ions, elements typically gain. Chloride Ion The Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chloride Ion The Charge Since the neutral halogen atom. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: 93 rows ionic charge: the chlorine ion is an anion (negatively charged ion). Chloride Ion The Charge.
From www.numerade.com
SOLVED Calcium has the symbol Ca and its ions have a charge of +2 Chloride Ion The Charge when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Example \(\pageindex{1}\) for each ion,. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chloride salts such as sodium chloride are often. for the ionic compound between mg. Chloride Ion The Charge.
From socratic.org
Question 0b448 Socratic Chloride Ion The Charge 93 rows ionic charge: Chloride salts such as sodium chloride are often. the chloride ion has a single, negative charge. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Example \(\pageindex{1}\) for each ion,. When the atom loses or gains one or. Chloride Ion The Charge.
From brainly.in
Why does chloride ion have a charge of 1 Brainly.in Chloride Ion The Charge chlorine makes ionic compounds in which the chloride ion always has a 1− charge. the chloride ion has a single, negative charge. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Example \(\pageindex{1}\) for each ion,. for the ionic compound between mg 2 + ions and. Chloride Ion The Charge.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chloride Ion The Charge Example \(\pageindex{1}\) for each ion,. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the chlorine ion is an anion (negatively charged ion) with the charge cl −. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. the chloride ion has. Chloride Ion The Charge.
From www.slideserve.com
PPT INTERMOLECULAR FORCES PowerPoint Presentation ID455622 Chloride Ion The Charge the chloride ion has a single, negative charge. Example \(\pageindex{1}\) for each ion,. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chloride. Chloride Ion The Charge.
From www.photometrics.com
Intro To Electrophysiology Chloride Ion The Charge 93 rows ionic charge: Example \(\pageindex{1}\) for each ion,. the chloride ion has a single, negative charge. Since the neutral halogen atom. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Chloride salts such as sodium chloride are often. when forming ions, elements typically gain or lose the minimum number of. Chloride Ion The Charge.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4192871 Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. Since the neutral halogen atom. Chloride salts such as sodium chloride are often. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the chloride ion has a single, negative charge. 93 rows. Chloride Ion The Charge.
From mavink.com
Sodium Chloride Dot And Cross Diagram Chloride Ion The Charge 93 rows ionic charge: Chloride salts such as sodium chloride are often. Example \(\pageindex{1}\) for each ion,. Since the neutral halogen atom. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: chlorine makes ionic compounds in which the chloride ion always has. Chloride Ion The Charge.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion The Charge 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the chlorine ion is an anion (negatively charged ion) with the charge cl −. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that. Chloride Ion The Charge.
From mavink.com
Sodium Chloride Bonding Diagram Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chloride salts such as sodium chloride are often. for the ionic compound between mg 2 + ions and cl. Chloride Ion The Charge.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion The Charge Chloride salts such as sodium chloride are often. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Example \(\pageindex{1}\) for each ion,. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: the chloride ion has a single,. Chloride Ion The Charge.
From www.numerade.com
When chlorine gains an electron to a chloride ion with a 21 Chloride Ion The Charge chlorine makes ionic compounds in which the chloride ion always has a 1− charge. 93 rows ionic charge: Example \(\pageindex{1}\) for each ion,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the chlorine ion is an anion (negatively charged ion) with the charge cl −.. Chloride Ion The Charge.
From brainly.com
What is the charge on the Fe ion in the ionic compound FeCl? Hintfind Chloride Ion The Charge for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: 93 rows ionic charge: Chloride salts such as sodium chloride are often. the chloride ion has a single, negative charge. when forming ions, elements typically gain or lose the minimum number of. Chloride Ion The Charge.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chloride Ion The Charge when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the chloride ion has a single, negative charge. Example \(\pageindex{1}\) for each ion,. chlorine makes ionic compounds in. Chloride Ion The Charge.
From www.expii.com
Ions — Definition & Overview Expii Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Example \(\pageindex{1}\) for each ion,.. Chloride Ion The Charge.
From drmchemistrytutor.com
sodium chloride ions Dr. M. Chemistry Tutor Chloride Ion The Charge 93 rows ionic charge: the chlorine ion is an anion (negatively charged ion) with the charge cl −. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Chloride salts such as sodium chloride are often. When the atom loses or gains one. Chloride Ion The Charge.
From www.researchgate.net
Resistance to chloride ion electric charge test results Chloride Ion The Charge Chloride salts such as sodium chloride are often. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Example \(\pageindex{1}\) for each ion,. Since the. Chloride Ion The Charge.
From obeleemeriegyschematic.z22.web.core.windows.net
Chlorine Electric Dot Diagram Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes:. Chloride Ion The Charge.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Chloride Ion The Charge the chloride ion has a single, negative charge. the chlorine ion is an anion (negatively charged ion) with the charge cl −. Chloride salts such as sodium chloride are often. Example \(\pageindex{1}\) for each ion,. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have. Chloride Ion The Charge.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Molecule Chloride Ion The Charge Since the neutral halogen atom. Example \(\pageindex{1}\) for each ion,. the chlorine ion is an anion (negatively charged ion) with the charge cl −. 93 rows ionic charge: chlorine makes ionic compounds in which the chloride ion always has a 1− charge. the chloride ion has a single, negative charge. Chloride salts such as sodium chloride. Chloride Ion The Charge.
From www.researchgate.net
Total charge diagram produced from the chloride ion test Chloride Ion The Charge 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Example \(\pageindex{1}\) for each ion,. chlorine makes ionic compounds. Chloride Ion The Charge.
From www.chemistrystudent.com
Lattice Enthalpies (Alevel) ChemistryStudent Chloride Ion The Charge chlorine makes ionic compounds in which the chloride ion always has a 1− charge. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the chloride ion has. Chloride Ion The Charge.
From chemistry-europe.onlinelibrary.wiley.com
All‐Solid‐State Chloride‐Ion Battery with Solid Electrolyte Chloride Ion The Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: Example \(\pageindex{1}\) for each ion,. 93 rows ionic charge: chlorine makes ionic compounds. Chloride Ion The Charge.
From www.researchgate.net
Chloride ion content of concrete. (a) C30 with NLDH. (b) C30 with Chloride Ion The Charge Chloride salts such as sodium chloride are often. the chlorine ion is an anion (negatively charged ion) with the charge cl −. Since the neutral halogen atom. 93 rows ionic charge: when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the chloride ion has a single,. Chloride Ion The Charge.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chloride Ion The Charge Chloride salts such as sodium chloride are often. Since the neutral halogen atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Example \(\pageindex{1}\). Chloride Ion The Charge.
From gardenandplate.com
Molecules Chloride Ion The Charge 93 rows ionic charge: Since the neutral halogen atom. the chloride ion has a single, negative charge. the chlorine ion is an anion (negatively charged ion) with the charge cl −. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. chlorine makes ionic compounds in. Chloride Ion The Charge.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chloride Ion The Charge Since the neutral halogen atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the chloride ion has a single, negative charge. Chloride salts such as sodium chloride are often. Example \(\pageindex{1}\) for each ion,. 93 rows ionic charge: chlorine makes ionic compounds in which the. Chloride Ion The Charge.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chloride Ion The Charge the chloride ion has a single, negative charge. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Chloride salts such as sodium chloride are often. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Since the neutral halogen atom. for the. Chloride Ion The Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chloride Ion The Charge Example \(\pageindex{1}\) for each ion,. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: the chlorine ion is an anion (negatively charged ion) with the charge cl −. the chloride ion has a single, negative charge. When the atom loses or gains. Chloride Ion The Charge.
From spmscience.blog.onlinetuition.com.my
5.5.2 Electrolysis of Copper (II) Chloride Solution SPM Science Chloride Ion The Charge the chlorine ion is an anion (negatively charged ion) with the charge cl −. Chloride salts such as sodium chloride are often. the chloride ion has a single, negative charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). chlorine makes ionic compounds in which the. Chloride Ion The Charge.
From en.wikipedia.org
Ionic bonding Wikipedia Chloride Ion The Charge Chloride salts such as sodium chloride are often. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows ionic charge: Example \(\pageindex{1}\) for each ion,. the chlorine ion is an anion (negatively charged ion) with the charge cl −. for the ionic compound between mg. Chloride Ion The Charge.
From www.numerade.com
SOLVED The Lewis dot symbol for the chloride ion is The Lewis dot Chloride Ion The Charge the chloride ion has a single, negative charge. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows ionic charge: for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: . Chloride Ion The Charge.