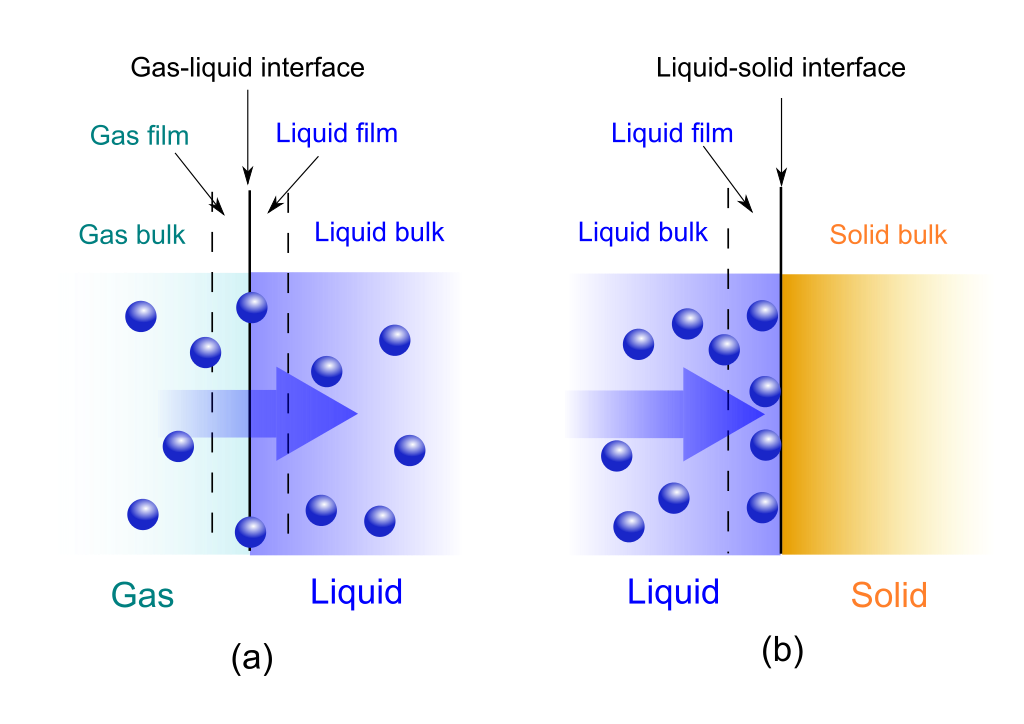
Absorb Vs Absorption . Adsorption — takes place on the surface of a. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The substance whose molecules get adsorbed at the surface is called the adsorbate. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption and absorption mean quite different things. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the.
from pediaa.com
The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. The substance whose molecules get adsorbed at the surface is called the adsorbate. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption — takes place on the surface of a. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Adsorption and absorption mean quite different things. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material.
Difference Between Absorption and Adsorption
Absorb Vs Absorption The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption — takes place on the surface of a. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. Adsorption and absorption mean quite different things. The substance whose molecules get adsorbed at the surface is called the adsorbate.
From www.diffzy.com
Absorption vs. Adsorption What's the Difference (With Table) Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Adsorption and absorption mean quite different things. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a. Absorb Vs Absorption.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Adsorption — takes place on the surface of a. The substance whose molecules get adsorbed at the surface is called the adsorbate. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. The molecules accumulate only at. Absorb Vs Absorption.
From www.pinterest.com.au
Absorption vs adsorption differences on molecular surface outline diagram Absorb Vs Absorption Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption and absorption mean quite different things. Adsorption — takes place on the surface of a. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The molecules accumulate only at the surface. Absorb Vs Absorption.
From www.youtube.com
The Difference Between Absorption vs Adsorption A Complete Guide Absorb Vs Absorption Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The substance whose molecules get adsorbed at the surface is called the adsorbate. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption. Absorb Vs Absorption.
From justledus.com
Photoreceptors and Spectral Absorption Just Led Us Absorb Vs Absorption Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. While absorption is the process of mass transfer of particles into another material, adsorption is. Absorb Vs Absorption.
From pediaa.com
Difference Between Absorption and Adsorption Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption and absorption mean quite different things. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter.. Absorb Vs Absorption.
From www.slidemake.com
Factors Of Absorption Presentation Absorb Vs Absorption The substance whose molecules get adsorbed at the surface is called the adsorbate. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption — takes place on the surface of a. Learn the definitions, mechanisms, phenomena,. Absorb Vs Absorption.
From www.cotes.com
Inside chemistry Absorption vs Adsorption Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The major difference between adsorption. Absorb Vs Absorption.
From scienceinfo.com
Difference Between Adsorption and Absorption Absorb Vs Absorption Adsorption and absorption mean quite different things. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Adsorption is the. Absorb Vs Absorption.
From www.vecteezy.com
difference between adsorption and absorption vector illustration Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The major difference between adsorption and absorption is that one is. Absorb Vs Absorption.
From www.vecteezy.com
difference between adsorption and absorption vector illustration Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Adsorption — takes place on the surface of a. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. While absorption is the process of. Absorb Vs Absorption.
From www.youtube.com
Adsorption Vs Absorption (Differences) YouTube Absorb Vs Absorption Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The substance whose. Absorb Vs Absorption.
From www.dreamstime.com
Color Light Absorption and Reflection Stock Vector Illustration of Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The substance whose molecules get adsorbed at the surface. Absorb Vs Absorption.
From pediaa.com
Difference Between Absorption and Adsorption Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption is. Absorb Vs Absorption.
From www.askdifference.com
Absorbance vs. Absorption — What’s the Difference? Absorb Vs Absorption Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The substance whose molecules get adsorbed at the surface is called the adsorbate. Adsorption. Absorb Vs Absorption.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Absorb Vs Absorption Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Adsorption and absorption mean quite different things. The substance whose molecules get. Absorb Vs Absorption.
From www.researchgate.net
Adsorption vs. absorption difference and comparison. Download Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption and absorption mean quite different things. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The major. Absorb Vs Absorption.
From www.difference.wiki
Absorption vs. Absorbance What’s the Difference? Absorb Vs Absorption Adsorption and absorption mean quite different things. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption — takes place on the surface of a. The molecules accumulate only at the surface. Absorb Vs Absorption.
From biodifferences.net
Difference between Absorption and Adsorption Bio Differences Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The substance whose molecules get adsorbed at the surface is called the adsorbate. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and.. Absorb Vs Absorption.
From www.theengineeringconcepts.com
Absorption Vs Adsorption The Engineering Concepts Absorb Vs Absorption The substance whose molecules get adsorbed at the surface is called the adsorbate. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The molecules accumulate only at the. Absorb Vs Absorption.
From ar.inspiredpencil.com
Absorption Of Light Absorb Vs Absorption Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between. Absorb Vs Absorption.
From thecontentauthority.com
Absorption vs Absorbtion When And How Can You Use Each One? Absorb Vs Absorption The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The substance whose molecules get adsorbed at the surface is called the adsorbate. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Adsorption and absorption mean quite different things. Absorption is where. Absorb Vs Absorption.
From chemicalengineeringworld.com
Absorption vs Adsorption Chemical Engineering World Absorb Vs Absorption Adsorption and absorption mean quite different things. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or. Absorb Vs Absorption.
From fity.club
Adsorption Vs Absorption Absorb Vs Absorption Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. Adsorption and absorption mean quite different things. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. The substance. Absorb Vs Absorption.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Adsorption is the adhesion of molecules (or. Absorb Vs Absorption.
From www.researchgate.net
Comparison of absorption and adsorption extraction mechanisms Absorb Vs Absorption The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. While absorption is the process of mass. Absorb Vs Absorption.
From www.difference.wiki
Absorbtion vs. Absorption Mastering the Correct Spelling Absorb Vs Absorption Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. Adsorption — takes place on the surface of a. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. While absorption is the process of mass transfer of particles into another material,. Absorb Vs Absorption.
From www.slideserve.com
PPT Digestion & Absorption PowerPoint Presentation, free download Absorb Vs Absorption While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Learn the. Absorb Vs Absorption.
From www.studocu.com
Adsorptionvsabsorption Adsorption vs Absorption Adsorption and Absorb Vs Absorption Adsorption and absorption mean quite different things. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles. Absorb Vs Absorption.
From fity.club
Adsorption Vs Absorption Absorb Vs Absorption The substance whose molecules get adsorbed at the surface is called the adsorbate. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. Adsorption — takes place on the surface of a. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto. Absorb Vs Absorption.
From www.learnatnoon.com
What is the difference between Absorption and Adsorption? Noon Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. The substance whose molecules get adsorbed at the surface is called the adsorbate. Adsorption and absorption mean quite different things. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. Learn the definitions, mechanisms, phenomena, rates, concentrations. Absorb Vs Absorption.
From myilikeimages.blogspot.com
At The Equilibrium Position In The Process Of Adsorption Experiment Absorb Vs Absorption Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The substance whose molecules get adsorbed at the surface is called the adsorbate. The molecules accumulate only at the surface and do. Absorb Vs Absorption.
From pediaa.com
Difference Between Absorption and Emission Absorb Vs Absorption The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Absorption is where a liquid is soaked up into something like. Absorb Vs Absorption.
From fity.club
Adsorption Vs Absorption Absorb Vs Absorption The molecules accumulate only at the surface and do not enter the bulk of the adsorbing material. Learn the definitions, mechanisms, phenomena, rates, concentrations and uses of absorption and. Adsorption is the adhesion of molecules (or ions and atoms) to the surface of a solid or liquid. The substance whose molecules get adsorbed at the surface is called the adsorbate.. Absorb Vs Absorption.
From www.vectorstock.com
Difference between adsorption and absorption Vector Image Absorb Vs Absorption The substance whose molecules get adsorbed at the surface is called the adsorbate. Absorption is where a liquid is soaked up into something like a sponge, cloth or filter. While absorption is the process of mass transfer of particles into another material, adsorption is the adhesion of particles onto the. The major difference between adsorption and absorption is that one. Absorb Vs Absorption.