Antacids Neutralize Acid By The Following Reaction . Identify the salt in the following equation: Antacids work by neutralizing stomach acid. Identify the salt in the following equation: Antacids work by counteracting (neutralising) the acid in your stomach. Despite the many commercial brand, almost all antacids act on. 3) antacids neutralize acid by the following reaction. Antacids are bases used to neutralize the acid that causes heartburn. The stomach has an acidic interior generated by dilute hcl,. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. They do this because the chemicals in antacids are. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Here's a breakdown of the reaction: Antacids neutralize acid by the following reaction. Mg(oh)2 + 2hcl → mgcl2 + h2o E) none of the answers is.
from scienceinfo.com
Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Identify the salt in the following equation: Antacids are bases used to neutralize the acid that causes heartburn. They do this because the chemicals in antacids are. Mg(oh)2 + 2hcl → mgcl2 + h2o. Here's a breakdown of the reaction: Identify the salt in the following equation: 3) antacids neutralize acid by the following reaction. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids work by counteracting (neutralising) the acid in your stomach.
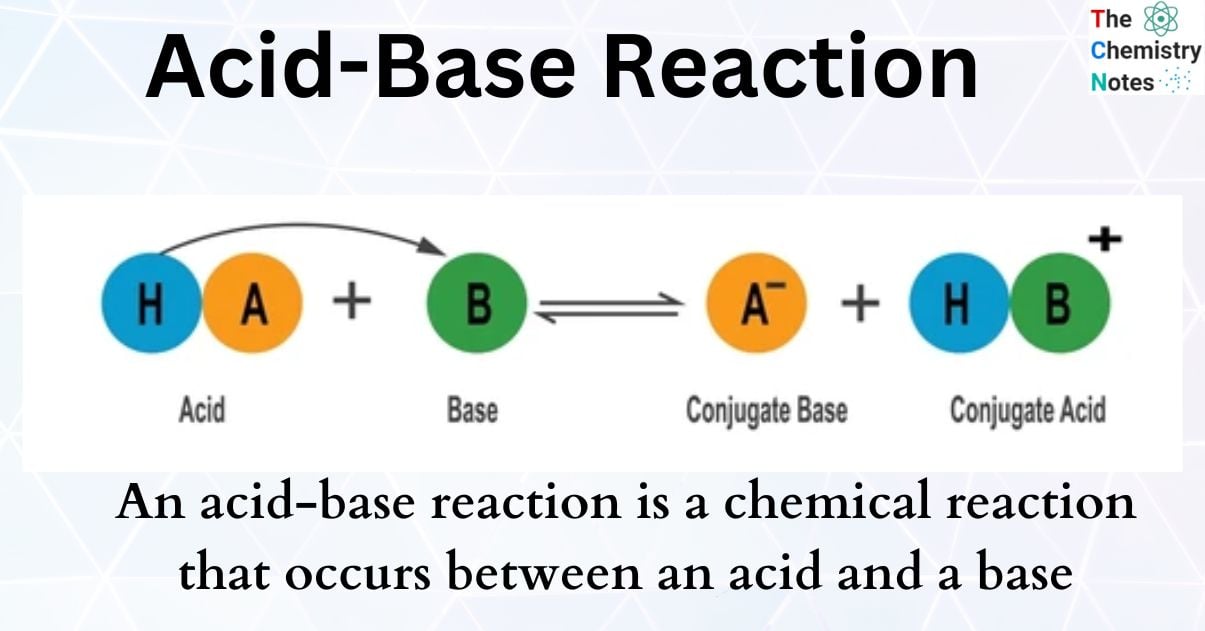
AcidBase Reaction (Neutralization Reaction)
Antacids Neutralize Acid By The Following Reaction Antacids are bases used to neutralize the acid that causes heartburn. They do this because the chemicals in antacids are. Despite the many commercial brand, almost all antacids act on. Antacids work by neutralizing stomach acid. Antacids are bases used to neutralize the acid that causes heartburn. Identify the salt in the following equation: 3) antacids neutralize acid by the following reaction. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Identify the salt in the following equation: Antacids work by counteracting (neutralising) the acid in your stomach. The stomach has an acidic interior generated by dilute hcl,. Mg(oh)2 + 2hcl → mgcl2 + h2o. E) none of the answers is. Mg(oh)2 + 2hcl → mgcl2 + h2o Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Antacids neutralize acid by the following reaction.
From www.numerade.com
SOLVED Antacids are used to treat acid reflux by neutralizing stomach Antacids Neutralize Acid By The Following Reaction In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids neutralize acid by the following reaction. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Here's a breakdown of the reaction: Mg(oh)2 + 2hcl → mgcl2 + h2o. Identify the salt in the. Antacids Neutralize Acid By The Following Reaction.
From www.teachoo.com
[MCQ] The graph given below depicts a neutralization reaction (acid Antacids Neutralize Acid By The Following Reaction Despite the many commercial brand, almost all antacids act on. They do this because the chemicals in antacids are. Antacids work by counteracting (neutralising) the acid in your stomach. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids are bases used to neutralize the acid that causes heartburn. E) none of. Antacids Neutralize Acid By The Following Reaction.
From scienceinfo.com
AcidBase Reaction (Neutralization Reaction) Antacids Neutralize Acid By The Following Reaction Antacids neutralize acid by the following reaction. Here's a breakdown of the reaction: Antacids work by neutralizing stomach acid. Mg(oh)2 + 2hcl → mgcl2 + h2o The stomach has an acidic interior generated by dilute hcl,. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids are bases used to neutralize the. Antacids Neutralize Acid By The Following Reaction.
From joiamsdzz.blob.core.windows.net
How To Neutralize Acid Water at Anne Fretwell blog Antacids Neutralize Acid By The Following Reaction Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. They do this because the chemicals in antacids are. 3) antacids neutralize acid by the following reaction. Antacids work by counteracting (neutralising) the acid in your stomach. They do this through a chemical reaction in which the antacid combines. Antacids Neutralize Acid By The Following Reaction.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Antacids Neutralize Acid By The Following Reaction Despite the many commercial brand, almost all antacids act on. Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Mg(oh)2 + 2hcl → mgcl2 + h2o. Here's a breakdown of the reaction: They do this through a chemical reaction in which the antacid combines with the acid in. Antacids Neutralize Acid By The Following Reaction.
From www.chegg.com
Solved Antacids neutralize the hydrochloric acid in your Antacids Neutralize Acid By The Following Reaction E) none of the answers is. Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Antacids are bases used to neutralize the acid that causes heartburn. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Mg(oh)2 + 2hcl → mgcl2. Antacids Neutralize Acid By The Following Reaction.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Antacids Neutralize Acid By The Following Reaction Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. They do this because the chemicals in antacids are. Despite the many commercial brand, almost all antacids act on. 3) antacids neutralize acid by the following reaction. Mg(oh)2 + 2hcl → mgcl2 + h2o E) none of the answers. Antacids Neutralize Acid By The Following Reaction.
From answermueller.z21.web.core.windows.net
Chemical Reaction In Acidbase Neutralization Antacids Neutralize Acid By The Following Reaction The stomach has an acidic interior generated by dilute hcl,. Antacids work by neutralizing stomach acid. Mg(oh)2 + 2hcl → mgcl2 + h2o Antacids neutralize acid by the following reaction. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Identify the salt in the following equation: Despite the many commercial brand,. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the neutralization Antacids Neutralize Acid By The Following Reaction Identify the salt in the following equation: They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Mg(oh)2 + 2hcl → mgcl2 + h2o. Antacids work by counteracting (neutralising) the acid in your stomach. The stomach has an acidic interior generated by dilute hcl,. Mg(oh)2 + 2hcl → mgcl2 + h2o 3). Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVED[Rovicm" Topics] [Referencas] Draw the structure(s) of the major Antacids Neutralize Acid By The Following Reaction 3) antacids neutralize acid by the following reaction. Antacids work by neutralizing stomach acid. Antacids are bases used to neutralize the acid that causes heartburn. Mg(oh)2 + 2hcl → mgcl2 + h2o Despite the many commercial brand, almost all antacids act on. Antacids work by counteracting (neutralising) the acid in your stomach. Identify the salt in the following equation: The. Antacids Neutralize Acid By The Following Reaction.
From slideplayer.com
2 Chemical Principles. ppt download Antacids Neutralize Acid By The Following Reaction Despite the many commercial brand, almost all antacids act on. The stomach has an acidic interior generated by dilute hcl,. Identify the salt in the following equation: E) none of the answers is. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Identify the salt in the following equation: Here's a. Antacids Neutralize Acid By The Following Reaction.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Antacids Neutralize Acid By The Following Reaction Antacids work by counteracting (neutralising) the acid in your stomach. Antacids work by neutralizing stomach acid. Identify the salt in the following equation: Mg(oh)2 + 2hcl → mgcl2 + h2o E) none of the answers is. Here's a breakdown of the reaction: They do this through a chemical reaction in which the antacid combines with the acid in the stomach.. Antacids Neutralize Acid By The Following Reaction.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry Antacids Neutralize Acid By The Following Reaction In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. 3) antacids neutralize acid by the following reaction. Identify the salt in the following equation: They do this because the chemicals in antacids are. Antacids are bases used to neutralize the acid that causes heartburn. Antacids work by counteracting (neutralising) the acid in. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVEDSome of the substances commonly used in stomach antacids are MgO Antacids Neutralize Acid By The Following Reaction Identify the salt in the following equation: They do this because the chemicals in antacids are. Antacids neutralize acid by the following reaction. Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is. Antacids Neutralize Acid By The Following Reaction.
From www.coursehero.com
[Solved] Antacids neutralize acid by the following reaction. Identify Antacids Neutralize Acid By The Following Reaction Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Despite the many commercial brand, almost all antacids act on. Identify the salt in the following equation: Antacids are bases used to neutralize the acid that causes heartburn. The stomach has an acidic interior generated by dilute hcl,. In. Antacids Neutralize Acid By The Following Reaction.
From www.slideshare.net
Antacids Antacids Neutralize Acid By The Following Reaction The stomach has an acidic interior generated by dilute hcl,. They do this because the chemicals in antacids are. Mg(oh)2 + 2hcl → mgcl2 + h2o In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Despite the many commercial brand, almost all antacids act on. They do this through a chemical reaction. Antacids Neutralize Acid By The Following Reaction.
From www.youtube.com
D.4 Antacids (SL) YouTube Antacids Neutralize Acid By The Following Reaction Antacids work by counteracting (neutralising) the acid in your stomach. The stomach has an acidic interior generated by dilute hcl,. Antacids are bases used to neutralize the acid that causes heartburn. Mg(oh)2 + 2hcl → mgcl2 + h2o Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. They. Antacids Neutralize Acid By The Following Reaction.
From www.coursehero.com
[Solved] Antacids neutralize acid by the following reaction. Identify Antacids Neutralize Acid By The Following Reaction Antacids neutralize acid by the following reaction. Identify the salt in the following equation: Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Mg(oh)2 + 2hcl → mgcl2 + h2o E) none of the answers is. Antacids work by counteracting (neutralising) the acid in your stomach. Mg(oh)2 +. Antacids Neutralize Acid By The Following Reaction.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID4269478 Antacids Neutralize Acid By The Following Reaction Mg(oh)2 + 2hcl → mgcl2 + h2o E) none of the answers is. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids neutralize acid by the following reaction. Identify the salt in the following equation: Mg(oh)2 + 2hcl → mgcl2 + h2o. They do this through a chemical reaction in which. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVED Part A Write the balanced chemical equation for the Antacids Neutralize Acid By The Following Reaction Identify the salt in the following equation: Mg(oh)2 + 2hcl → mgcl2 + h2o. Despite the many commercial brand, almost all antacids act on. Antacids work by neutralizing stomach acid. E) none of the answers is. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Mg(oh)2 + 2hcl → mgcl2 +. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
Antacids are compounds that neutralize stomach ac… Antacids Neutralize Acid By The Following Reaction Despite the many commercial brand, almost all antacids act on. Antacids are bases used to neutralize the acid that causes heartburn. They do this because the chemicals in antacids are. Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. 3) antacids neutralize acid by the following reaction. Antacids. Antacids Neutralize Acid By The Following Reaction.
From slideplayer.com
Acids and Bases. ppt download Antacids Neutralize Acid By The Following Reaction In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids neutralize acid by the following reaction. The stomach has an acidic interior generated by dilute hcl,. They do this because the chemicals in antacids are. 3) antacids neutralize acid by the following reaction. Despite the many commercial brand, almost all antacids act. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVED Antacids neutralize acid by the following reaction (10 point Antacids Neutralize Acid By The Following Reaction Identify the salt in the following equation: Mg(oh)2 + 2hcl → mgcl2 + h2o Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. E) none of the answers is. 3) antacids. Antacids Neutralize Acid By The Following Reaction.
From worksheetlistkastner.z1.web.core.windows.net
Acid Base Neutralization Reaction Worksheet Antacids Neutralize Acid By The Following Reaction The stomach has an acidic interior generated by dilute hcl,. Here's a breakdown of the reaction: Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Antacids work by counteracting (neutralising) the acid in your stomach. Identify the salt in the following equation: Mg(oh)2 + 2hcl → mgcl2 +. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVEDAntacids are bases that neutralize acids in the digestive tract Antacids Neutralize Acid By The Following Reaction Despite the many commercial brand, almost all antacids act on. They do this because the chemicals in antacids are. Antacids neutralize acid by the following reaction. Mg(oh)2 + 2hcl → mgcl2 + h2o The stomach has an acidic interior generated by dilute hcl,. Identify the salt in the following equation: 3) antacids neutralize acid by the following reaction. E) none. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVED Antacids neutralize stomach acids. Using a suitable equation Antacids Neutralize Acid By The Following Reaction The stomach has an acidic interior generated by dilute hcl,. Antacids neutralize acid by the following reaction. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. Antacids work by counteracting (neutralising) the acid in your stomach. Antacids work by neutralizing stomach acid. Identify the salt in the following equation: 3) antacids neutralize. Antacids Neutralize Acid By The Following Reaction.
From slideplayer.com
Chapter 2 Chemical Principles. ppt download Antacids Neutralize Acid By The Following Reaction Antacids work by neutralizing stomach acid. 3) antacids neutralize acid by the following reaction. Mg(oh)2 + 2hcl → mgcl2 + h2o. In the given reaction, mg (oh)₂ + 2hcl → mgcl₂ + h₂o, the salt produced is mgcl₂. The stomach has an acidic interior generated by dilute hcl,. They do this through a chemical reaction in which the antacid combines. Antacids Neutralize Acid By The Following Reaction.
From studylib.net
Lab Neutralization Antacids Neutralize Acid By The Following Reaction Antacids work by neutralizing stomach acid. They do this because the chemicals in antacids are. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. The stomach has an acidic interior generated by dilute hcl,. Despite the many commercial brand, almost all antacids act on. Mg(oh)2 + 2hcl → mgcl2 + h2o.. Antacids Neutralize Acid By The Following Reaction.
From lessonfulljean.z13.web.core.windows.net
Complete The Following Acid Base Reaction Antacids Neutralize Acid By The Following Reaction Antacids neutralize acid by the following reaction. Mg(oh)2 + 2hcl → mgcl2 + h2o Here's a breakdown of the reaction: 3) antacids neutralize acid by the following reaction. The stomach has an acidic interior generated by dilute hcl,. They do this because the chemicals in antacids are. They do this through a chemical reaction in which the antacid combines with. Antacids Neutralize Acid By The Following Reaction.
From www.bartleby.com
Answered Antacids neutralize the hydrochloric… bartleby Antacids Neutralize Acid By The Following Reaction Mg(oh)2 + 2hcl → mgcl2 + h2o. Despite the many commercial brand, almost all antacids act on. E) none of the answers is. Antacids work by neutralizing stomach acid. They do this because the chemicals in antacids are. Identify the salt in the following equation: The stomach has an acidic interior generated by dilute hcl,. Mg(oh)2 + 2hcl → mgcl2. Antacids Neutralize Acid By The Following Reaction.
From www.flinnsci.ca
Neutralization Reaction of an Antacid Flinn Scientific Antacids Neutralize Acid By The Following Reaction 3) antacids neutralize acid by the following reaction. Despite the many commercial brand, almost all antacids act on. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. The stomach has an acidic interior generated by dilute hcl,. Antacids are bases used to neutralize the acid that causes heartburn. They do this. Antacids Neutralize Acid By The Following Reaction.
From worksheetdbointed.z13.web.core.windows.net
Acid Base Neutralization Reactions Worksheet Antacids Neutralize Acid By The Following Reaction Identify the salt in the following equation: They do this because the chemicals in antacids are. Antacids are bases used to neutralize the acid that causes heartburn. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids work by counteracting (neutralising) the acid in your stomach. Identify the salt in the. Antacids Neutralize Acid By The Following Reaction.
From aznswerzonelisunanchored.z13.web.core.windows.net
Explain A Neutralization Reaction Antacids Neutralize Acid By The Following Reaction Mg(oh)2 + 2hcl → mgcl2 + h2o They do this because the chemicals in antacids are. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Mg(oh)2 + 2hcl → mgcl2 + h2o. Identify the salt in the following equation: Antacids work by neutralizing stomach acid. Antacids work by counteracting (neutralising) the. Antacids Neutralize Acid By The Following Reaction.
From www.numerade.com
SOLVED Some of the substances commonly used in stomach antacids are Antacids Neutralize Acid By The Following Reaction Once the naoh solution has been standardized, it can be used to determine the acid neutralizing capacity of an antacid tablet. Here's a breakdown of the reaction: Antacids work by counteracting (neutralising) the acid in your stomach. The stomach has an acidic interior generated by dilute hcl,. Mg(oh)2 + 2hcl → mgcl2 + h2o. Despite the many commercial brand, almost. Antacids Neutralize Acid By The Following Reaction.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Antacids Neutralize Acid By The Following Reaction They do this because the chemicals in antacids are. Antacids work by neutralizing stomach acid. The stomach has an acidic interior generated by dilute hcl,. Identify the salt in the following equation: Antacids work by counteracting (neutralising) the acid in your stomach. Antacids are bases used to neutralize the acid that causes heartburn. Mg(oh)2 + 2hcl → mgcl2 + h2o. Antacids Neutralize Acid By The Following Reaction.