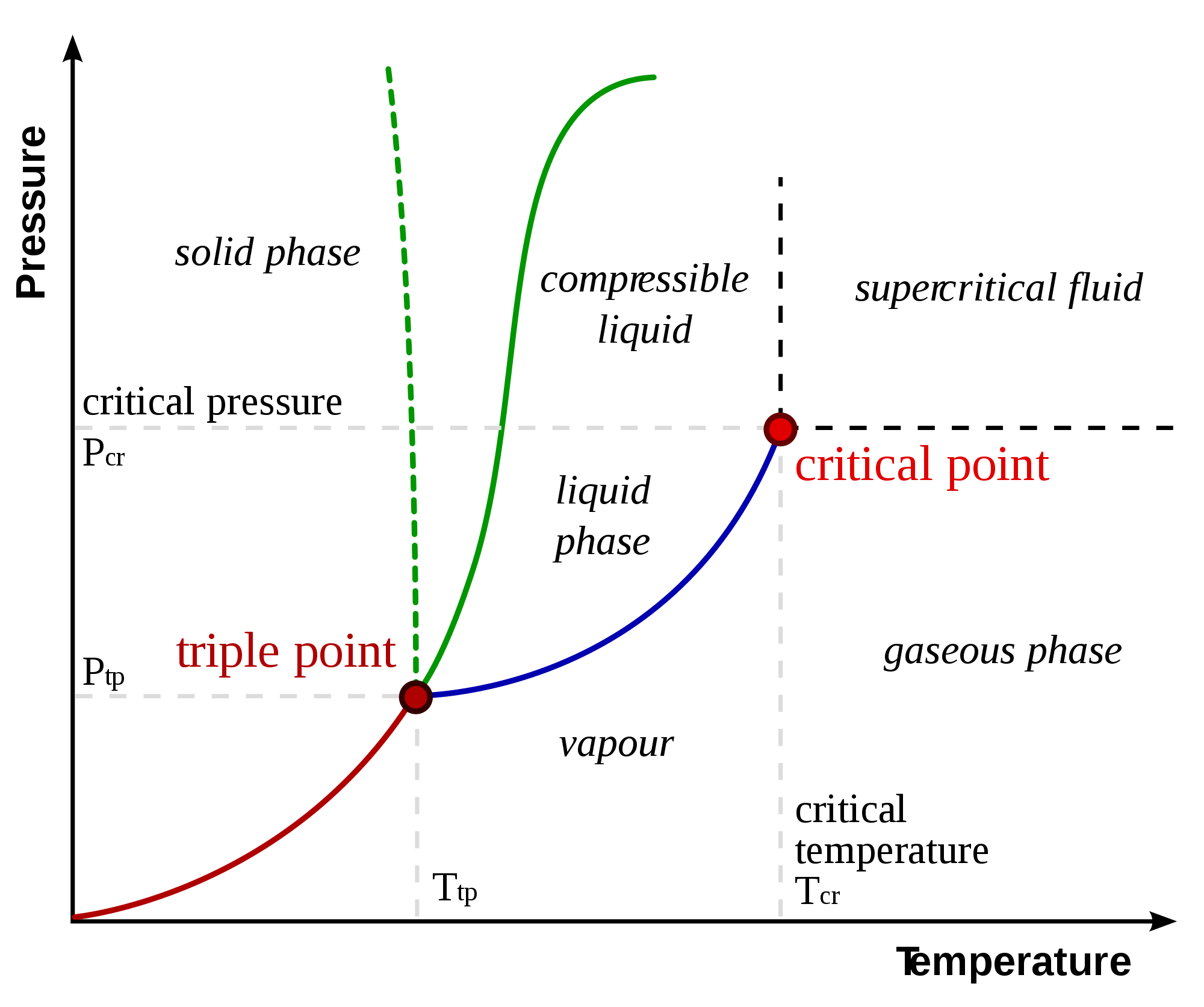
Example Of Equilibrium Reaction In Solid Liquid And Gas Phase . Understand the types of physical equilibrium i.e. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Two key points are worth emphasizing. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. An example of this is adding glucose (sugar) to water, where so much sugar is added. Physical equilibrium develops between different phases or physical properties. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. In most cases the dissolved gas will undergo a reaction in the liquid phase.
from byjus.com
Two key points are worth emphasizing. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. In most cases the dissolved gas will undergo a reaction in the liquid phase. An example of this is adding glucose (sugar) to water, where so much sugar is added.
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law
Example Of Equilibrium Reaction In Solid Liquid And Gas Phase When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. In most cases the dissolved gas will undergo a reaction in the liquid phase. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Two key points are worth emphasizing. An example of this is adding glucose (sugar) to water, where so much sugar is added.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. Two key points are worth emphasizing. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. In most cases the dissolved gas will undergo a reaction in the liquid phase. An example of this is adding glucose. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From learningschoolboyer.z13.web.core.windows.net
Show The Phase Change Diagram Process Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. In most cases the dissolved gas will undergo a reaction in the liquid phase. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. The equilibrium is between. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. In most cases the dissolved gas will undergo a reaction in the. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.coursehero.com
Solid to Gas Phase Transition Introduction to Chemistry Course Hero Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. Two key points are worth emphasizing. Understand the types of physical equilibrium i.e. An example of this is adding glucose (sugar) to water, where so much sugar is added. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Systems. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Example Of Equilibrium Reaction In Solid Liquid And Gas Phase When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. In most cases the dissolved gas will undergo a reaction in the liquid phase. Two key points are worth emphasizing. Understand the types of physical equilibrium i.e. An example of this is adding glucose (sugar) to water, where. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Two key points are worth emphasizing. An example of this is adding glucose (sugar) to water, where so much sugar is added. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid.. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From exymotkoo.blob.core.windows.net
Solid And Liquid Phases In Equilibrium at Jessica Florian blog Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. Two key points are worth emphasizing. An example of this is adding glucose (sugar) to water, where so much sugar is added. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Removing heat from a substance changes a. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Two key points are worth emphasizing. The equilibrium is between the undissolved solid. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From studylib.net
Equilibrium for a general reaction Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Understand the types of physical equilibrium i.e. An example of this is adding glucose (sugar) to water, where so much sugar is added. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. The equilibrium is between. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Equilibrium Basic Concepts PowerPoint Presentation, free download Example Of Equilibrium Reaction In Solid Liquid And Gas Phase An example of this is adding glucose (sugar) to water, where so much sugar is added. Understand the types of physical equilibrium i.e. In most cases the dissolved gas will undergo a reaction in the liquid phase. Physical equilibrium develops between different phases or physical properties. Removing heat from a substance changes a gas to a liquid, or a liquid. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Understand the types of physical equilibrium i.e. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Two key points are. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.youtube.com
GasPhase Equilibrium (Example) YouTube Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. An example of this is adding glucose (sugar) to water, where so much sugar is added. Physical equilibrium develops between different phases or physical properties. In most cases the dissolved gas will undergo a reaction in the liquid phase. Understand the types of. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From exymotkoo.blob.core.windows.net
Solid And Liquid Phases In Equilibrium at Jessica Florian blog Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Understand the types of physical equilibrium i.e. Physical equilibrium develops between different phases or physical properties. Two key points are worth emphasizing. In most cases the dissolved gas will undergo a reaction in the liquid phase. An example of this is adding glucose (sugar) to water, where so much sugar is added. When the products and reactants of an equilibrium. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From unistudium.unipg.it
Phase Diagrams Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. An example of this is adding glucose (sugar) to water, where so much sugar is added. Two key. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From shaunmwilliams.com
Chapter 1 Presentation Example Of Equilibrium Reaction In Solid Liquid And Gas Phase An example of this is adding glucose (sugar) to water, where so much sugar is added. Two key points are worth emphasizing. Understand the types of physical equilibrium i.e. Physical equilibrium develops between different phases or physical properties. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From exymotkoo.blob.core.windows.net
Solid And Liquid Phases In Equilibrium at Jessica Florian blog Example Of Equilibrium Reaction In Solid Liquid And Gas Phase When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Physical equilibrium develops between different phases or physical properties. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Physical equilibrium develops between different phases or physical properties. An example of this is adding glucose (sugar) to water, where so. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT 10. Equilibrium State of a Gas Phase Reaction PowerPoint Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Understand the types of physical equilibrium i.e. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Physical equilibrium develops between different phases or physical properties. In most cases the dissolved gas will undergo a reaction in the. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Two key points are worth emphasizing. An example of this is adding glucose (sugar) to water, where so much sugar is added. In most cases the dissolved gas will undergo a reaction in the liquid phase. Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. When the products and reactants of an equilibrium. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Understand the types of physical equilibrium i.e. Two key points are worth emphasizing. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT 10. Equilibrium State of a Gas Phase Reaction PowerPoint Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. In most cases the dissolved gas will undergo a reaction in the liquid phase. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Physical equilibrium develops between different phases or physical properties. Two key points are. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT 10. Equilibrium State of a Gas Phase Reaction PowerPoint Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. Two key points are worth emphasizing. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. An example of this is adding glucose (sugar) to water, where so much sugar is added. Systems where no reaction occurs are known as. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Example Of Equilibrium Reaction In Solid Liquid And Gas Phase The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. An example of this is adding glucose (sugar) to water, where so much sugar is added. Understand the types of physical equilibrium i.e. Physical equilibrium develops between different phases or physical properties. Removing heat from a substance changes a gas to a liquid, or. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Understand the types of physical equilibrium i.e. In most cases the dissolved gas will undergo a reaction in the liquid phase. Two key points are worth emphasizing. When. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From learncheme.com
gasphasechemicalequilibriumsummary LearnChemE Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. In most cases the dissolved gas will undergo a reaction in the liquid phase. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Removing heat from a substance changes a gas to a liquid, or a liquid. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From saylordotorg.github.io
Chemical Equilibrium Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Two key points are worth emphasizing. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Physical equilibrium develops between different. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Physical equilibrium develops between different phases or physical properties. Understand the. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Ch. 13 Chemical Equilibrium PowerPoint Presentation, free Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. An example of this is adding glucose (sugar) to water, where so much sugar is added. Two key points are worth emphasizing. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Removing heat from a substance changes. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Understand the types of physical equilibrium i.e. When the products and reactants of. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From byjus.com
What is the name of the process when a liquid changes into a gas? Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Physical equilibrium develops between different phases or physical properties. An example of this. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Example Of Equilibrium Reaction In Solid Liquid And Gas Phase An example of this is adding glucose (sugar) to water, where so much sugar is added. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Physical equilibrium develops between different phases or physical properties. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation Example Of Equilibrium Reaction In Solid Liquid And Gas Phase In most cases the dissolved gas will undergo a reaction in the liquid phase. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. Removing heat from a substance changes a gas to a liquid, or a liquid. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From chem.libretexts.org
Chapter 11.7 Phase Diagrams Chemistry LibreTexts Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. Understand the types of physical equilibrium i.e. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. An example of this. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Example Of Equilibrium Reaction In Solid Liquid And Gas Phase Systems where no reaction occurs are known as physical absorption while the systems where reaction occurs. Two key points are worth emphasizing. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid. In most cases the dissolved gas will undergo a reaction in the liquid phase. Physical equilibrium develops between different phases or. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.
From www.youtube.com
GasPhase Equilibrium Conversion Part 2 YouTube Example Of Equilibrium Reaction In Solid Liquid And Gas Phase An example of this is adding glucose (sugar) to water, where so much sugar is added. When the products and reactants of an equilibrium reaction form a single phase, whether gas or liquid, the system is a homogeneous. Physical equilibrium develops between different phases or physical properties. Removing heat from a substance changes a gas to a liquid, or a. Example Of Equilibrium Reaction In Solid Liquid And Gas Phase.