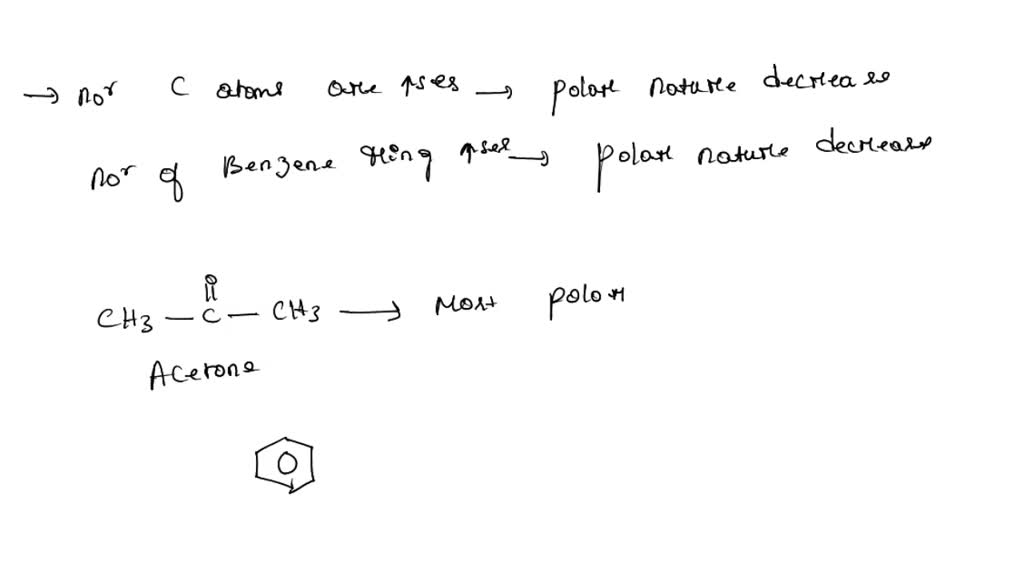
How Is Acetone Polar . Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is said to be a polar. Is acetone polar or nonpolar?
from www.numerade.com
Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone is said to be a polar. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Is acetone polar or nonpolar? However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less.
SOLVED List the following from most polar to least polar Acetone
How Is Acetone Polar Acetone is said to be a polar. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone is said to be a polar. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule.
From chemistry.stackexchange.com
organic chemistry Why bond energy of acetone is more though it is How Is Acetone Polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Is acetone polar or nonpolar? Because of the carbonyl group , acetone is a somewhat polar molecule. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a. How Is Acetone Polar.
From loetqglys.blob.core.windows.net
Is Acetone Polar Or Nonpolar at Deborah Simon blog How Is Acetone Polar Is acetone polar or nonpolar? Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. There are different degrees of polarity, and acetone is less polar than water,. Acetone is said to be a polar. Because of the carbonyl group , acetone is a somewhat polar. How Is Acetone Polar.
From www.youtube.com
Acetone Lewis Structure How to Draw the Lewis Structure for Acetone How Is Acetone Polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Because of the carbonyl group , acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Acetone, one of the principal ketone bodies elevated during. How Is Acetone Polar.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Is acetone polar or nonpolar? Because of the carbonyl group , acetone is a somewhat. How Is Acetone Polar.
From it.wikipedia.org
Acetone Wikipedia How Is Acetone Polar Acetone is said to be a polar. There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. However,. How Is Acetone Polar.
From www.specialbutikken.dk
Polar acetone 5L How Is Acetone Polar Acetone is said to be a polar. There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Because of the carbonyl group , acetone is a somewhat polar. How Is Acetone Polar.
From www.numerade.com
SOLVEDpolar aprotic solvents, like acetone, can only solvate cations How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Acetone is said to be a polar. However, acetone. How Is Acetone Polar.
From carlynewsturner.blogspot.com
Acetone Polar or Nonpolar Solvent How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. However, acetone is still considered a polar aprotic solvent,. How Is Acetone Polar.
From www.youtube.com
Is acetone polar or nonpolar? Will NaCl dissolve in it? YouTube How Is Acetone Polar Because of the carbonyl group , acetone is a somewhat polar molecule. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. There are different degrees of polarity, and acetone is less polar than water,. However, acetone is still considered a polar aprotic solvent, despite the. How Is Acetone Polar.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar How Is Acetone Polar Because of the carbonyl group , acetone is a somewhat polar molecule. Is acetone polar or nonpolar? Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone is said to be a polar. Acetone, one of the principal ketone bodies elevated during treatment with the. How Is Acetone Polar.
From www.chegg.com
Solved Common polar solvents Water (H20) Acetone (CH3COCH3) How Is Acetone Polar Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Acetone is said to be a polar. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Because of the carbonyl group , acetone is. How Is Acetone Polar.
From www.youtube.com
Intermolecular Forces for (CH3)2CO Acetone YouTube How Is Acetone Polar Acetone is said to be a polar. Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Unsymmetrical geometry,. How Is Acetone Polar.
From ehandel.mw.dk
POLAR ACETONE 1 LTR M. Wilhelmsen A/S How Is Acetone Polar There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone is said to be a polar. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure. How Is Acetone Polar.
From www.chegg.com
Solved 1. Acetone is a polar aprotic solvent (structure How Is Acetone Polar Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Is acetone polar or nonpolar? However, acetone is still. How Is Acetone Polar.
From www.researchgate.net
The FTIR spectra of acetonesoluble and acetoneinsoluble fractions of How Is Acetone Polar Is acetone polar or nonpolar? However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is said to be a polar. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone, one of. How Is Acetone Polar.
From www.pinterest.com
SN2 or SN1 polar aprotic and polar protic solvents a Organic How Is Acetone Polar Acetone is said to be a polar. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. There are different degrees of polarity, and acetone. How Is Acetone Polar.
From chemistrymolecule.blogspot.com
Chemistry Molecule Acetone How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection. How Is Acetone Polar.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution How Is Acetone Polar Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are. How Is Acetone Polar.
From www.specialbutikken.dk
Polar acetone 5L How Is Acetone Polar There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. However, acetone is still considered a polar aprotic solvent, despite the fact that it is. How Is Acetone Polar.
From www.chegg.com
Solved An electrostatic potential map for acetone (CH3)2CO), How Is Acetone Polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Is acetone polar or nonpolar? Acetone is said to be a polar.. How Is Acetone Polar.
From www.youtube.com
Is C3H6O Polar or NonPolar? (Acetone) YouTube How Is Acetone Polar Because of the carbonyl group , acetone is a somewhat polar molecule. Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone, one of the principal ketone. How Is Acetone Polar.
From loetqglys.blob.core.windows.net
Is Acetone Polar Or Nonpolar at Deborah Simon blog How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Because of the carbonyl group , acetone is a somewhat polar molecule. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is said. How Is Acetone Polar.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity How Is Acetone Polar Because of the carbonyl group , acetone is a somewhat polar molecule. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Is acetone polar or nonpolar? However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. How Is Acetone Polar.
From brainly.com
indicate polar bonds for acetone. draw a vector representing the How Is Acetone Polar Because of the carbonyl group , acetone is a somewhat polar molecule. Is acetone polar or nonpolar? Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Acetone is said to be a polar. Unsymmetrical geometry, the nature of the bonds, and the. How Is Acetone Polar.
From www.youtube.com
Why does acetone dissolve both polar and nonpolar? YouTube How Is Acetone Polar Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone is said to be a polar. However, acetone is still considered a polar aprotic solvent, despite the fact that it. How Is Acetone Polar.
From www.numerade.com
SOLVED List the following from most polar to least polar Acetone How Is Acetone Polar Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. There are different degrees of polarity, and acetone is less polar than water,. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that. How Is Acetone Polar.
From www.chegg.com
Solved Will acetone dissolve in benzene? Benzene's molecular How Is Acetone Polar Acetone is said to be a polar. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Is acetone polar or nonpolar? Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone, one of the principal ketone bodies elevated during treatment with the. How Is Acetone Polar.
From loetqglys.blob.core.windows.net
Is Acetone Polar Or Nonpolar at Deborah Simon blog How Is Acetone Polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. There are different degrees of polarity, and acetone is less polar than water,. Acetone is said to be a polar. Because of the carbonyl group , acetone is a somewhat polar molecule. Is acetone polar or nonpolar? Acetone, one. How Is Acetone Polar.
From www.chegg.com
Solved Given Acetone, (CH3)2CO, is a widely used industrial How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Because of the carbonyl group , acetone is a somewhat polar molecule. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone, one of. How Is Acetone Polar.
From www.researchgate.net
Polarizability spectra for water, acetone, and ethanol. Each of the How Is Acetone Polar There are different degrees of polarity, and acetone is less polar than water,. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Is acetone polar or nonpolar? Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make. How Is Acetone Polar.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity How Is Acetone Polar Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar molecule. Acetone is said to be a polar. However,. How Is Acetone Polar.
From www.youtube.com
Is C3H6O (Acetone) Polar or NonPolar? YouTube How Is Acetone Polar Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Acetone is said to be a polar. Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group , acetone is a somewhat polar. How Is Acetone Polar.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? How Is Acetone Polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by the. Is acetone polar or nonpolar? There are different degrees of polarity, and. How Is Acetone Polar.
From www.dmtv.dk
Polar Polar acetone 2,5 L DMT Værktøj How Is Acetone Polar There are different degrees of polarity, and acetone is less polar than water,. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone, one of the principal ketone bodies elevated during treatment with the ketogenic diet, exhibits anticonvulsant properties that may contribute to the seizure protection conferred by. How Is Acetone Polar.
From www.techno-science.net
Acétone Définition et Explications How Is Acetone Polar There are different degrees of polarity, and acetone is less polar than water,. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar. Because of the carbonyl group , acetone is a somewhat polar molecule. However, acetone is still considered a polar aprotic solvent, despite the. How Is Acetone Polar.