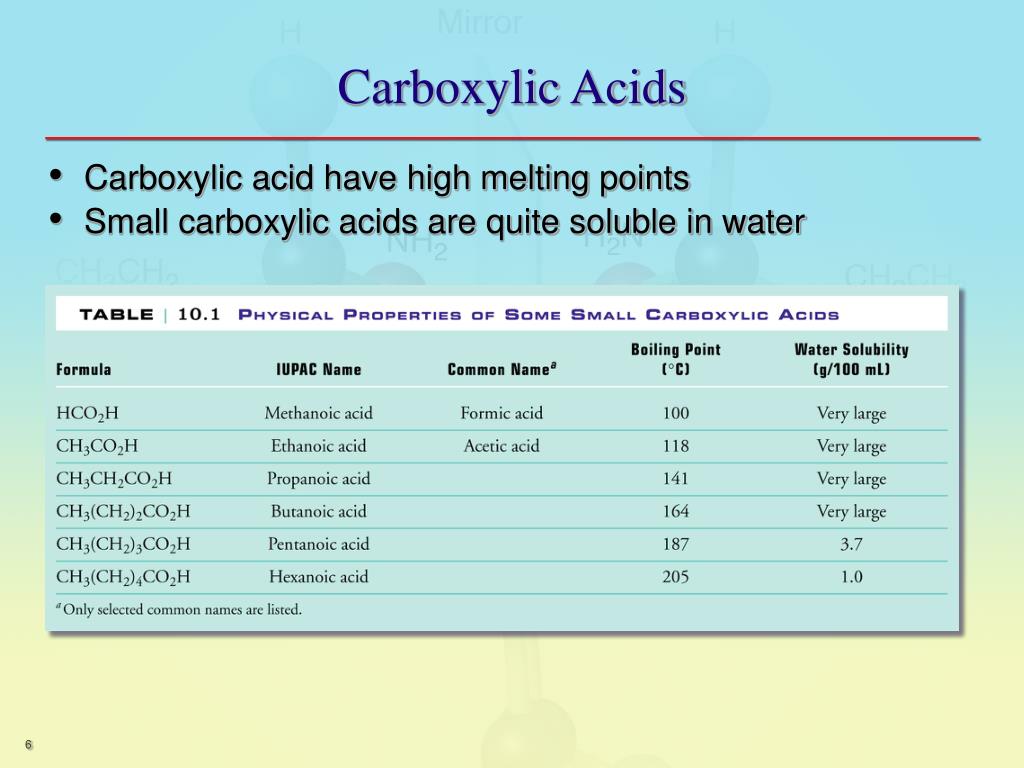
Amino Acids Have High Melting Point . The amino acids are crystalline solids with surprisingly high melting points. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. Their other properties varying for each particular amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively.
from www.slideserve.com
The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. It is difficult to pin the melting points down exactly because. Their other properties varying for each particular amino acid. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. In addition, amino acids are amphiprotic;
PPT Chem 150 Unit 7 Organic Molecules II Carboxylic Acids, Phenols
Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because. The amino acids are crystalline solids with surprisingly high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Their other properties varying for each particular amino acid.
From www.slideserve.com
PPT Chem 150 Unit 7 Organic Molecules II Carboxylic Acids, Phenols Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Account for some of the typical properties of. Amino Acids Have High Melting Point.
From slidetodoc.com
Amino Acids Proteins and Enzymes Types of Proteins Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; The amino acids are crystalline solids with surprisingly high melting points. Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. They have large. Amino Acids Have High Melting Point.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. Their other properties varying for each particular amino acid. The amino acids are crystalline solids with surprisingly high melting points. In addition, amino acids are amphiprotic; Account for some. Amino Acids Have High Melting Point.
From www.chegg.com
Solved s. سلام OH + H2N. ОН NH2 amino acid (methionine) Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. Their other properties varying for each particular amino. Amino Acids Have High Melting Point.
From www.researchgate.net
Melting points and enthalpies of individual fatty acids Download Table Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. The amino acids are crystalline solids with surprisingly high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are. Amino Acids Have High Melting Point.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6534192 Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. In addition, amino acids are amphiprotic; The amino. Amino Acids Have High Melting Point.
From www.numerade.com
SOLVED When protein contains two Or more chains folded together; it is Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because. The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. The amino acids are. Amino Acids Have High Melting Point.
From brainly.in
Amino acids are found to have high melting point and solubility in Amino Acids Have High Melting Point It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. It is difficult to pin the melting points down exactly because. The amino acids are crystalline solids with surprisingly high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in. Amino Acids Have High Melting Point.
From exobfetmk.blob.core.windows.net
Why Do Amino Acids Have High Melting Point at Colin Aleman blog Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. The amino acids are crystalline solids with surprisingly high melting points. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with. Amino Acids Have High Melting Point.
From byjus.com
The melting points and solubility in water of amino acids are generally Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. Account for some of the typical properties of amino. Amino Acids Have High Melting Point.
From slideplayer.com
Examples of Lipids. ppt download Amino Acids Have High Melting Point It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. The. Amino Acids Have High Melting Point.
From www.numerade.com
SOLVED 03. (8 Marks) Write the main diffcrcncels betwcct thc following Amino Acids Have High Melting Point Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. In addition, amino acids are amphiprotic; Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. It. Amino Acids Have High Melting Point.
From www.numerade.com
SOLVED Q3. (8 Marks) Write the main differences between the following Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. In addition, amino acids are amphiprotic; Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. It. Amino Acids Have High Melting Point.
From jesus-well-salazar.blogspot.com
Double Bonds in Fatty Acids Melting Point Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. In addition, amino acids are amphiprotic; The. Amino Acids Have High Melting Point.
From brainly.in
why do amino acids have high melting point and are soluble in water Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. They have large dipole moments, are relatively soluble in. Amino Acids Have High Melting Point.
From brainly.com
Rank the following fatty acids a coording to their melting point, from Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. The amino acids are crystalline solids with surprisingly high melting points. Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively. Amino Acids Have High Melting Point.
From leah4sci.com
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. The amino acids are crystalline solids with surprisingly high melting points. In addition, amino acids are amphiprotic; Their other properties varying for each particular amino acid. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and. Amino Acids Have High Melting Point.
From www.numerade.com
SOLVED What is the difference between protein digestion and protein Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. The. Amino Acids Have High Melting Point.
From quizlet.com
Diagram of Melting Points of Fatty Acids Diagram Quizlet Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. The amino acids are crystalline solids with surprisingly high melting points. In addition, amino acids are amphiprotic; Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble. Amino Acids Have High Melting Point.
From www.researchgate.net
DSC assay of the 21 amino acid studied and the relative melting point Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. It is difficult to pin the melting points down exactly because. In addition, amino acids are amphiprotic; They have. Amino Acids Have High Melting Point.
From www.chegg.com
Solved Arrange the fatty acids in order of increasing Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. Their other properties varying for each particular amino acid. They have large dipole moments, are relatively soluble in water but insoluble. Amino Acids Have High Melting Point.
From www.brainkart.com
Amino Acids Can Act as Both Acids and Bases Amino Acids Have High Melting Point Their other properties varying for each particular amino acid. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. It is difficult to pin the melting points down exactly. Amino Acids Have High Melting Point.
From matmake.com
Melting Point of Amino Acids Table Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with. Amino Acids Have High Melting Point.
From exobfetmk.blob.core.windows.net
Why Do Amino Acids Have High Melting Point at Colin Aleman blog Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. It is. Amino Acids Have High Melting Point.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because. In addition, amino acids are. Amino Acids Have High Melting Point.
From www.chegg.com
Solved Arrange the fatty acids from highest melting point to Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. The amino acids are crystalline solids with surprisingly high melting points. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms. Amino Acids Have High Melting Point.
From www.doubtnut.com
alphaAmino acids behave as crystalline ionic solid and have high melt Amino Acids Have High Melting Point Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. In addition, amino acids are amphiprotic; It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. They have. Amino Acids Have High Melting Point.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Amino Acids Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. Their other properties varying for each particular amino acid. The amino acids are crystalline solids with surprisingly high melting points. The amino acids are crystalline solids with surprisingly high melting points. They. Amino Acids Have High Melting Point.
From www.researchgate.net
DSC assay [N c ] of the 21 amino acids and their relative DSC melting Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. They have large dipole. Amino Acids Have High Melting Point.
From www.onlinebiologynotes.com
Properties of amino acids physical and chemical Online Biology Notes Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. It is difficult to pin the melting points down exactly because. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are. Amino Acids Have High Melting Point.
From www.youtube.com
alphaAmino acids behave as crystalline ionic solid and have high Amino Acids Have High Melting Point In addition, amino acids are amphiprotic; Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The amino acids are crystalline solids with surprisingly high melting points. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Their other. Amino Acids Have High Melting Point.
From www.chegg.com
Solved Rank The Following Fatty Acids From Highest Meltin... Amino Acids Have High Melting Point It is difficult to pin the melting points down exactly because. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. Their other properties varying for each particular amino acid. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. Account. Amino Acids Have High Melting Point.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Amino Acids Have High Melting Point They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively. It is difficult to pin the melting points down exactly because. In addition, amino acids are amphiprotic; Their other properties varying for each particular amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility. Amino Acids Have High Melting Point.
From www.doubtnut.com
The melting points of amino acids are higher than the corresponding ha Amino Acids Have High Melting Point It is difficult to pin the melting points down exactly because. Their other properties varying for each particular amino acid. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high melting points. The amino acids are crystalline solids with surprisingly high melting points. In addition, amino acids are amphiprotic; Account. Amino Acids Have High Melting Point.
From ericvisser.nl
Melting point chart Ericvisser Amino Acids Have High Melting Point The amino acids are crystalline solids with surprisingly high melting points. Their other properties varying for each particular amino acid. It is difficult to pin the melting points down exactly because the amino acids tend to decompose before they melt. They have large dipole moments, are relatively soluble in water but insoluble in hydrocarbons, and are crystalline with relatively high. Amino Acids Have High Melting Point.