Bromine Reaction Definition . The general reaction can be represented as: when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. chemical reactions of bromine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. C = c + b. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the.
from www.slideserve.com
The general reaction can be represented as: One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. C = c + b. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. chemical reactions of bromine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The robust oxidizing properties of bromine are responsible for many of its chemical reactions.
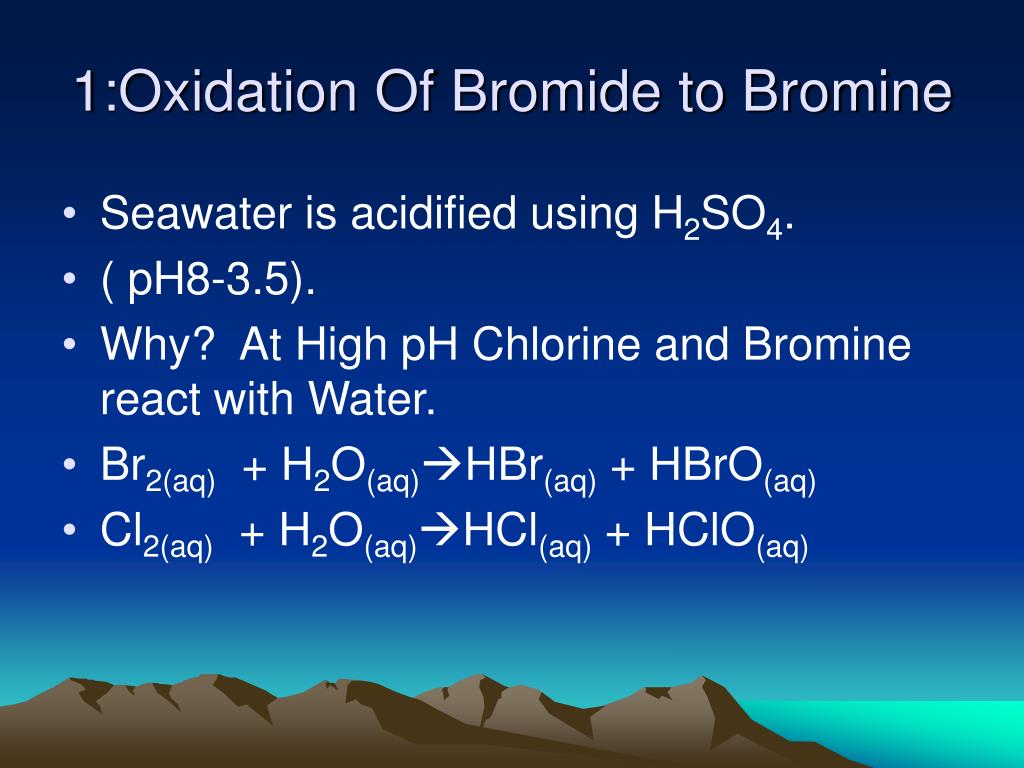
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185
Bromine Reaction Definition The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The general reaction can be represented as: One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. chemical reactions of bromine. C = c + b. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the.
From www.numerade.com
SOLVED On a microscopic level, how does the reaction of bromine with a saturated hydrocarbon Bromine Reaction Definition bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. C = c + b. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most. Bromine Reaction Definition.
From study.com
Bromination of Acetanilide Mechanism & Steps Lesson Bromine Reaction Definition the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. chemical reactions of bromine. bromination is a chemical. Bromine Reaction Definition.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID318185 Bromine Reaction Definition The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. chemical reactions of bromine. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine readily reacts. Bromine Reaction Definition.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Reaction Definition bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. chemical reactions of bromine. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The general reaction. Bromine Reaction Definition.
From www.youtube.com
Reaction of Phenol With Bromine Alcohols, Phenols and Ethers Chemistry Class 12 YouTube Bromine Reaction Definition bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. C = c + b. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The electron affinity of bromine is intermediate between those of. Bromine Reaction Definition.
From www.youtube.com
Neutralization of Bromine Beautiful Reaction YouTube Bromine Reaction Definition The general reaction can be represented as: bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other. Bromine Reaction Definition.
From www.researchgate.net
Bromine reactions included in the model. Download Table Bromine Reaction Definition bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. The general reaction can be represented as: chemical reactions of bromine. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. the product of the reaction is a dilute solution of bromine, from. Bromine Reaction Definition.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity Bromine Reaction Definition The general reaction can be represented as: C = c + b. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The robust oxidizing properties of bromine are responsible for. Bromine Reaction Definition.
From byjus.com
Explain the reaction mechanism of addition of bromine on acetylene by equations ? Tell the Bromine Reaction Definition when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. The general reaction can be represented as: The robust oxidizing properties of bromine are responsible for many of its chemical reactions.. Bromine Reaction Definition.
From www.sciencephoto.com
Bromine reaction Stock Image A500/0458 Science Photo Library Bromine Reaction Definition when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. C = c + b. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. . Bromine Reaction Definition.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity Bromine Reaction Definition One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The electron affinity of bromine is intermediate between those of chlorine and iodine,. Bromine Reaction Definition.
From pubs.acs.org
Reactivity of Bromine Radical with Dissolved Organic Matter Moieties and Monochloramine Effect Bromine Reaction Definition the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. chemical reactions of bromine. The general reaction can be represented as: when reacting with other elements, bromine typically forms ionic compounds with. Bromine Reaction Definition.
From www.slideserve.com
PPT Edexcel organic reaction mechanisms PowerPoint Presentation, free download ID3936128 Bromine Reaction Definition bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The general reaction can be represented as: the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. C. Bromine Reaction Definition.
From www.researchgate.net
Simplified scheme of bromine explosion and ozone destruction reactions... Download Scientific Bromine Reaction Definition bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. chemical reactions. Bromine Reaction Definition.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine to bromate and bromide Bromine Reaction Definition chemical reactions of bromine. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The general reaction can be represented as: the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The electron affinity of bromine is intermediate between those. Bromine Reaction Definition.
From www.masterorganicchemistry.com
What is Allylic Bromination? Master Organic Chemistry Bromine Reaction Definition bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. when reacting with other. Bromine Reaction Definition.
From www.researchgate.net
BromineLithium Exchange in 1 and 2 with Download Scientific Diagram Bromine Reaction Definition C = c + b. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. The robust oxidizing properties of. Bromine Reaction Definition.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Reaction Definition C = c + b. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its. Bromine Reaction Definition.
From www.expii.com
SingleReplacement Reactions — Definition & Examples Expii Bromine Reaction Definition The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. chemical reactions of bromine.. Bromine Reaction Definition.
From www.researchgate.net
(a) Simplified scheme showing the formation of reactive bromine species... Download Scientific Bromine Reaction Definition The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. bromination is a chemical reaction involving the reaction of a compound,. Bromine Reaction Definition.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity Bromine Reaction Definition bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. C = c + b. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its. Bromine Reaction Definition.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Reaction Definition the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine readily reacts with alkenes in. Bromine Reaction Definition.
From solvedlib.com
Consider the reaction of bromine with the pictured al… SolvedLib Bromine Reaction Definition when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. The general reaction can be represented. Bromine Reaction Definition.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Reaction Definition The robust oxidizing properties of bromine are responsible for many of its chemical reactions. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. One of the most fundamental. Bromine Reaction Definition.
From www.slideserve.com
PPT Reactions of alkenes PowerPoint Presentation, free download ID3089339 Bromine Reaction Definition One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. chemical reactions of bromine. The general reaction can be represented as: bromination is a chemical reaction involving the reaction of a compound, and bromine. Bromine Reaction Definition.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Chemistry Bromine Reaction Definition The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine readily reacts with alkenes in an addition reaction to form dibromoalkanes. bromination is a chemical reaction involving the reaction of a compound,. Bromine Reaction Definition.
From joelgordon.photoshelter.com
Reaction of bromine with an unsaturated hydrocarbon Joel Gordon Photography Bromine Reaction Definition chemical reactions of bromine. bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium.. Bromine Reaction Definition.
From exofhdgch.blob.core.windows.net
Bromine Ionic Or Covalent at Donna McMillian blog Bromine Reaction Definition when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. The general reaction can be represented as: bromination is a chemical reaction involving the reaction of a compound, and bromine. Bromine Reaction Definition.
From www.youtube.com
Electrophilic Addition Mechanism with Bromine YouTube Bromine Reaction Definition The general reaction can be represented as: One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The robust oxidizing properties of bromine are responsible for many of its chemical reactions.. Bromine Reaction Definition.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity Bromine Reaction Definition chemical reactions of bromine. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. C = c + b. The general reaction can be represented as: The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical. Bromine Reaction Definition.
From www.slideserve.com
PPT 7. Alkenes Reactions and Synthesis PowerPoint Presentation ID298383 Bromine Reaction Definition The general reaction can be represented as: the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. chemical reactions of bromine. bromine readily reacts with alkenes in an addition. Bromine Reaction Definition.
From fphoto.photoshelter.com
science exothermic reaction sodium bromine Fundamental Photographs The Art of Science Bromine Reaction Definition C = c + b. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The general reaction can be represented as: the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The electron affinity of bromine is intermediate. Bromine Reaction Definition.
From exoaayemj.blob.core.windows.net
Bromine Test Equation at John Cortese blog Bromine Reaction Definition C = c + b. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. The electron affinity of bromine is intermediate between those of chlorine and iodine, as. Bromine Reaction Definition.
From www.researchgate.net
Bromination reaction representation (17). Download Scientific Diagram Bromine Reaction Definition when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. the product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. One of the most fundamental. Bromine Reaction Definition.
From www.youtube.com
Bromine Addition Mechanism to Fumaric Acid Formation of meso bromine addition products Bromine Reaction Definition The general reaction can be represented as: when reacting with other elements, bromine typically forms ionic compounds with metals and covalent compounds with nonmetals. One of the most fundamental and straightforward reactions of bromine is with alkali metals like sodium. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other. Bromine Reaction Definition.