Which Element Has The Highest Shielding Effect . the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Because the 4f electrons are poorly. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron.
from mungfali.com
the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. This is because its nucleus has a very powerful attractive. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Because the 4f electrons are poorly. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons.
What Is Shielding Effect
Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. Because the 4f electrons are poorly. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Engineering Projects Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of.. Which Element Has The Highest Shielding Effect.
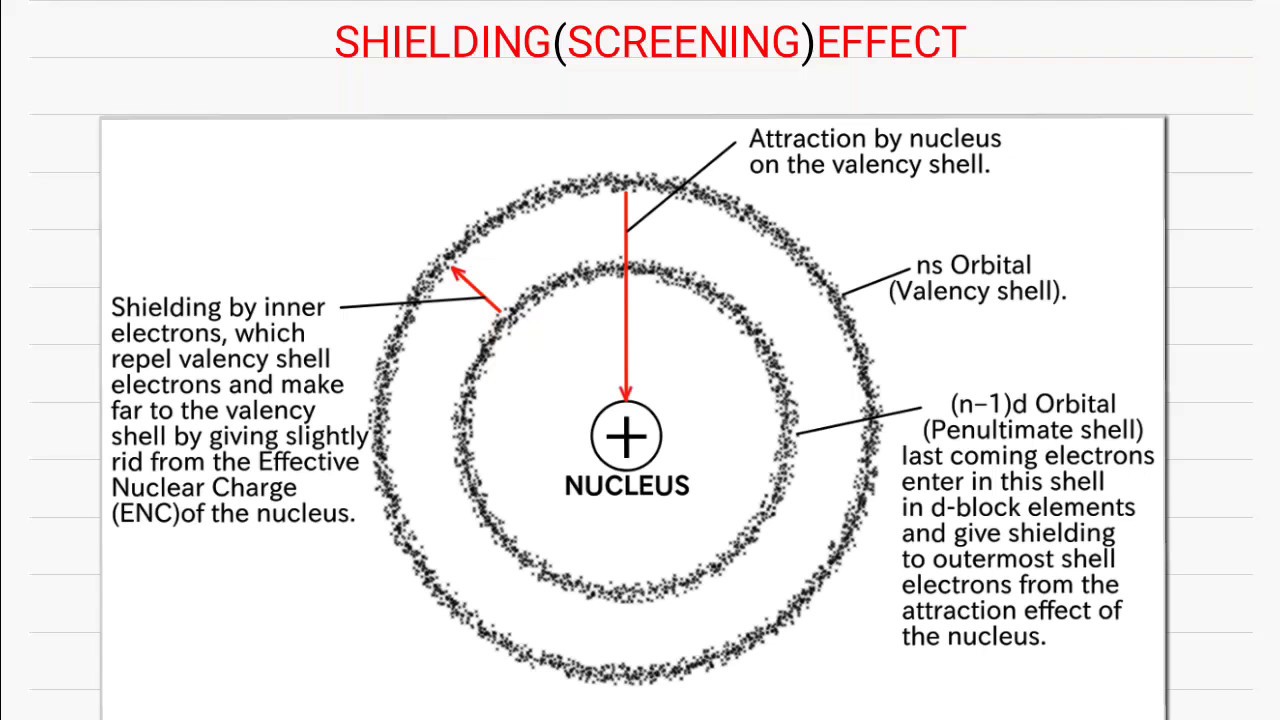
From exowuasqn.blob.core.windows.net
What Is Shielding Effect For Class 9 at Loretta Bryan blog Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear. Which Element Has The Highest Shielding Effect.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Which Element Has The Highest Shielding Effect the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This is because its nucleus has a very powerful. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 Which Element Has The Highest Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Because the 4f electrons are poorly. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the lanthanide contraction is the result of. Which Element Has The Highest Shielding Effect.
From sciencestruck.com
Shielding Effect Science Struck Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the lanthanide contraction is the result of the poor shielding effect of the. Which Element Has The Highest Shielding Effect.
From www.breakingatom.com
Shielding Which Element Has The Highest Shielding Effect the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0.. Which Element Has The Highest Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting, and 5 Reliable Applications Which Element Has The Highest Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. Because the 4f electrons are poorly. the concept of electron shielding, in which intervening electrons. Which Element Has The Highest Shielding Effect.
From www.breakingatom.com
Shielding of the Nucleus Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. fluorine (f) has the highest electronegativity on the whole. Which Element Has The Highest Shielding Effect.
From socratic.org
How are shielding effect and atomic radius related? Socratic Which Element Has The Highest Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Because the 4f electrons are poorly. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. This is because its nucleus has a very powerful attractive. the shielding effect describes the balance between the. Which Element Has The Highest Shielding Effect.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across A Period at Cynthia Which Element Has The Highest Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID118383 Which Element Has The Highest Shielding Effect the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Because the 4f electrons are poorly. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces. Which Element Has The Highest Shielding Effect.
From pt.slideshare.net
Periodic trends Which Element Has The Highest Shielding Effect the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Because the 4f electrons are poorly. fluorine (f) has the highest electronegativity on the whole periodic table with a score of. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free download ID5923721 Which Element Has The Highest Shielding Effect the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. Because the 4f electrons are poorly. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced. Which Element Has The Highest Shielding Effect.
From www.doubtnut.com
Which of the following element has highest shielding constant Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in. Which Element Has The Highest Shielding Effect.
From mungfali.com
What Is Shielding Effect Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the. Which Element Has The Highest Shielding Effect.
From www.allthescience.org
What Is the Shielding Effect? (with pictures) Which Element Has The Highest Shielding Effect the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the shielding effect describes the balance between the pull of the protons on valence electrons and. Which Element Has The Highest Shielding Effect.
From www.doubtnut.com
Which of the following element has highest shielding constant Which Element Has The Highest Shielding Effect This is because its nucleus has a very powerful attractive. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Because the 4f electrons are poorly. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electron shielding refers. Which Element Has The Highest Shielding Effect.
From zandex.org
What is shielding effect with example? Zandex Which Element Has The Highest Shielding Effect This is because its nucleus has a very powerful attractive. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. . Which Element Has The Highest Shielding Effect.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Which Element Has The Highest Shielding Effect fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept. Which Element Has The Highest Shielding Effect.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Engineering Projects Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the number of protons in the nucleus. Which Element Has The Highest Shielding Effect.
From exowuasqn.blob.core.windows.net
What Is Shielding Effect For Class 9 at Loretta Bryan blog Which Element Has The Highest Shielding Effect fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the number. Which Element Has The Highest Shielding Effect.
From thechemistrynotes.com
Shielding effect Which Element Has The Highest Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z. Which Element Has The Highest Shielding Effect.
From www.numerade.com
SOLVED which element would have the greatest shielding effect a.Na b.H c.Al d.Cd e.Bi Which Element Has The Highest Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Because the 4f electrons are poorly. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the number of protons in the nucleus is always shielded by the inner shells, weakening. Which Element Has The Highest Shielding Effect.
From cedzwiqd.blob.core.windows.net
Which Element Has The Most Shielding Electrons at Johnny Clark blog Which Element Has The Highest Shielding Effect electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the number of protons in the nucleus is always shielded by the inner shells, weakening. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT Periodic Patterns PowerPoint Presentation, free download ID2877545 Which Element Has The Highest Shielding Effect fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. This is because its nucleus has a very powerful attractive. Effective nuclear charge, zeff, experienced by an electron is less than the actual. Which Element Has The Highest Shielding Effect.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. This is because its nucleus has a very powerful attractive. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion. Which Element Has The Highest Shielding Effect.
From mungfali.com
What Is Shielding Effect Which Element Has The Highest Shielding Effect the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Because the 4f electrons are poorly. This is because its nucleus has a very powerful attractive. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. electron shielding. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID6734591 Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. This is because its nucleus has a very powerful attractive. Effective nuclear charge,. Which Element Has The Highest Shielding Effect.
From slidetodoc.com
Shielding Effect The shielding effect is the reduction Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. Because the 4f electrons are poorly. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Effective nuclear charge, zeff, experienced by an. Which Element Has The Highest Shielding Effect.
From www.numerade.com
SOLVED Which of the following elements with exhibit the greatest shielding effect Which Element Has The Highest Shielding Effect Because the 4f electrons are poorly. This is because its nucleus has a very powerful attractive. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. . Which Element Has The Highest Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table Which Element Has The Highest Shielding Effect fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. This is because its nucleus has a very powerful attractive. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electron shielding refers to the blocking of valence shell electron attraction by the nucleus,. Which Element Has The Highest Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting, and 5 Reliable Applications Which Element Has The Highest Shielding Effect This is because its nucleus has a very powerful attractive. fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. Which Element Has The Highest Shielding Effect.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID6840776 Which Element Has The Highest Shielding Effect fluorine (f) has the highest electronegativity on the whole periodic table with a score of 4.0. Because the 4f electrons are poorly. the number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. electron shielding refers to the blocking of valence shell electron attraction. Which Element Has The Highest Shielding Effect.
From www.youtube.com
What is Shielding Effect in Simple Words Shielding Effect Definition, Examples , Trend YouTube Which Element Has The Highest Shielding Effect the shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows. Which Element Has The Highest Shielding Effect.
From www.youtube.com
What does higher shielding effect mean Which element has the highest shielding effect? YouTube Which Element Has The Highest Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the lanthanide contraction is the result of the poor shielding effect of the 4f electrons.. Which Element Has The Highest Shielding Effect.