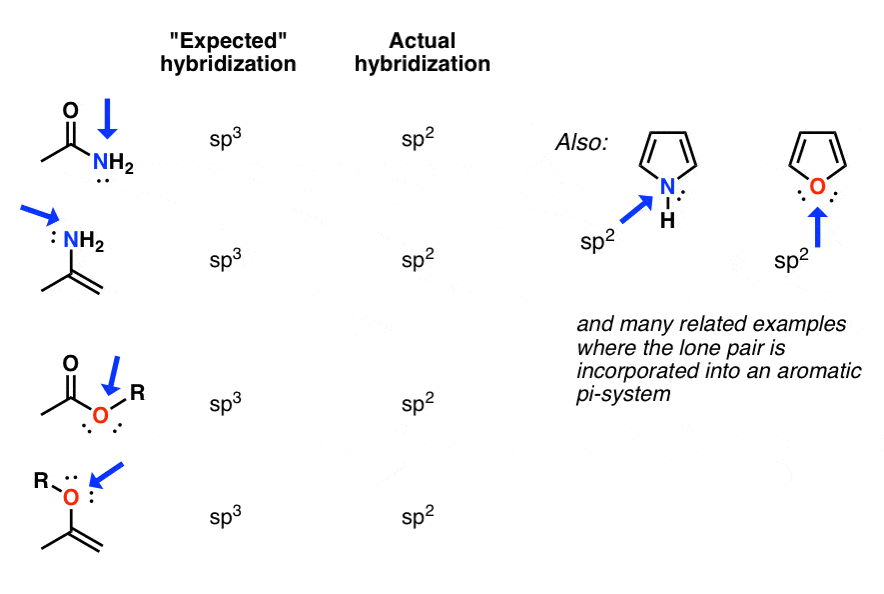
Hybridization Nitrogen . the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. For example, sp 3 hybridization for nitrogen results in. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. The molecular structure of water is. Note that, in this course, the term “lone pair” is used to. Sigma bond is the first bond that is made with other atoms.
from
Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. For example, sp 3 hybridization for nitrogen results in. The molecular structure of water is. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Sigma bond is the first bond that is made with other atoms. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon.
Hybridization Nitrogen For example, sp 3 hybridization for nitrogen results in. The molecular structure of water is. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Sigma bond is the first bond that is made with other atoms. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. For example, sp 3 hybridization for nitrogen results in. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair.
From
Hybridization Nitrogen Sigma bond is the first bond that is made with other atoms. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol. Hybridization Nitrogen.
From www.youtube.com
Organic Chemistry 1 Hybridization of nitrogen YouTube Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. Sigma bond is the first bond that is made with other atoms. The molecular structure of water is. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing. Hybridization Nitrogen.
From
Hybridization Nitrogen Note that, in this course, the term “lone pair” is used to. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. The molecular structure of water is. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. For example, sp 3. Hybridization Nitrogen.
From www.chegg.com
Solved 4. A) What is the hybridization of the nitrogen atom Hybridization Nitrogen For example, sp 3 hybridization for nitrogen results in. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. The molecular structure of water is. the hybridization schemes for nitrogen and. Hybridization Nitrogen.
From
Hybridization Nitrogen — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. The molecular structure of water is. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. Note that, in this course, the term “lone pair” is used to. . Hybridization Nitrogen.
From www.studocu.com
Chemistry 1 SP3 hybridized nitrogen atom structure Organic Hybridization Nitrogen The molecular structure of water is. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to. Hybridization Nitrogen.
From
Hybridization Nitrogen the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. Sigma bond is the first bond that is made with other atoms. The molecular structure of water is. For example, sp 3 hybridization for nitrogen results in. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen. Hybridization Nitrogen.
From
Hybridization Nitrogen like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. For example, sp 3. Hybridization Nitrogen.
From
Hybridization Nitrogen like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. For example, sp 3 hybridization for nitrogen results in. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Sigma bond is the first bond that is made with other atoms.. Hybridization Nitrogen.
From www.youtube.com
What is the hybridization of nitrogen in the azide ion ? 12 Hybridization Nitrogen — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. For example, sp 3 hybridization for nitrogen results in. Sigma bond is the first bond that is made with other atoms. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen. Hybridization Nitrogen.
From
Hybridization Nitrogen in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. The molecular structure of water is. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to. Hybridization Nitrogen.
From
Hybridization Nitrogen For example, sp 3 hybridization for nitrogen results in. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. — hybridization of nitrogen (n2) there are two types of bonds. Hybridization Nitrogen.
From
Hybridization Nitrogen For example, sp 3 hybridization for nitrogen results in. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to.. Hybridization Nitrogen.
From www.doubtnut.com
Hybridization of nitrogen atom in pyridine is Hybridization Nitrogen — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Sigma. Hybridization Nitrogen.
From
Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. the hybridization schemes for nitrogen and oxygen follow. Hybridization Nitrogen.
From
Hybridization Nitrogen in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Sigma bond is the first bond that is made with other atoms. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. the nitrogen atom is sp 3 hybridized with one hybrid. Hybridization Nitrogen.
From www.youtube.com
Hybridization of Nitrogen sp3 sp2 and sp Hybridization of Nitrogen Hybridization Nitrogen the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds.. Hybridization Nitrogen.
From
Hybridization Nitrogen the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. Note that, in this course, the term “lone pair” is used to. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. — hybridization of nitrogen (n2) there are two types of bonds which are. Hybridization Nitrogen.
From whatsinsight.org
N2 Lewis Structure Hybridization & Molecular Geometry What's Insight Hybridization Nitrogen Sigma bond is the first bond that is made with other atoms. The molecular structure of water is. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. Note that, in this course, the term “lone pair” is used to. like the carbon atom in. Hybridization Nitrogen.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Nitrogen Sigma bond is the first bond that is made with other atoms. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. The molecular structure of water is. For example, sp 3 hybridization for nitrogen results in. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for. Hybridization Nitrogen.
From
Hybridization Nitrogen like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. Note that, in this course, the term “lone pair” is used to. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. Nitrogen will also. Hybridization Nitrogen.
From www.youtube.com
What is the hybridization of the nitrogen atoms in N2 N2 Hybridization Nitrogen The molecular structure of water is. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Note that, in this course, the term “lone pair” is used to. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. —. Hybridization Nitrogen.
From
Hybridization Nitrogen — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. The molecular structure of water is. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the. Hybridization Nitrogen.
From www.numerade.com
SOLVED 7. What is the hybridization of the nitrogen atom in CH2NCH2CH3 Hybridization Nitrogen — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. For example, sp 3 hybridization for nitrogen results in. like the carbon atom in methane and the. Hybridization Nitrogen.
From
Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Sigma bond is the first bond that is made with other atoms. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. The molecular structure of water is. For example, sp 3 hybridization for nitrogen. Hybridization Nitrogen.
From www.chegg.com
Solved What is the hybridization of the indicated nitrogen Hybridization Nitrogen the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Note that, in this course, the term “lone pair” is used to. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by. Hybridization Nitrogen.
From
Hybridization Nitrogen Note that, in this course, the term “lone pair” is used to. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. For example, sp 3 hybridization for nitrogen results in. . Hybridization Nitrogen.
From
Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. Note that, in this course, the term “lone pair” is used to. The molecular structure of water is. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. Sigma bond is the first. Hybridization Nitrogen.
From www.chegg.com
Solved What is the hybridization of the indicated nitrogen Hybridization Nitrogen For example, sp 3 hybridization for nitrogen results in. Sigma bond is the first bond that is made with other atoms. Note that, in this course, the term “lone pair” is used to. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. — hybridization of nitrogen (n2) there are two types of bonds. Hybridization Nitrogen.
From
Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. For example, sp 3 hybridization for nitrogen results in. the hybridization schemes for nitrogen and oxygen follow the same guidelines. Hybridization Nitrogen.
From www.numerade.com
SOLVED The hybridization of nitrogen and oxygen atoms in the following Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. Note that, in this course, the term “lone. Hybridization Nitrogen.
From
Hybridization Nitrogen Sigma bond is the first bond that is made with other atoms. the nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form. Hybridization Nitrogen.
From
Hybridization Nitrogen Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. —. Hybridization Nitrogen.
From www.chegg.com
Solved What is the hybridization of nitrogen in the figure Hybridization Nitrogen The molecular structure of water is. Nitrogen will also hybridize sp 2 when there are only two atoms bonded to the nitrogen (one single. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the. Hybridization Nitrogen.
From
Hybridization Nitrogen like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol (methyl alcohol) and. For example, sp 3 hybridization for nitrogen results in. — hybridization of nitrogen (n2) there are two types of bonds which are widely used in chemistry, sigma (σ) and pi (π) bonds. The molecular structure of water is.. Hybridization Nitrogen.