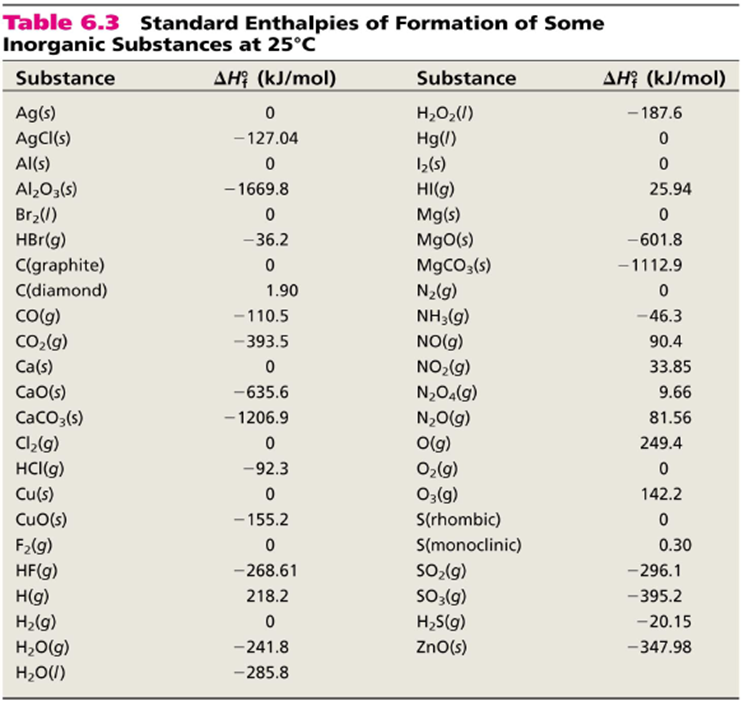
Standard Enthalpy Of Formation Reaction . using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. 193 rows — standard enthalpy of formation. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
from www.chem.fsu.edu
standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. 193 rows — standard enthalpy of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.
CHM1045 Enthalpy Lecture
Standard Enthalpy Of Formation Reaction the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. 193 rows — standard enthalpy of formation. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. a standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Enthalpy Of Formation Reaction.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Reaction the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows — standard enthalpy of formation. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of formation, also known as the heat of formation, is. Standard Enthalpy Of Formation Reaction.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Reaction a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. . Standard Enthalpy Of Formation Reaction.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Reaction standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy. Standard Enthalpy Of Formation Reaction.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Reaction standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for. Standard Enthalpy Of Formation Reaction.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Reaction the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. using the values in the above table of standard enthalpies of formation, calculate. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
6.9 Determining Enthalpies of Reaction from Standard Enthalpies of Standard Enthalpy Of Formation Reaction In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. 193 rows — standard enthalpy of formation. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of. Standard Enthalpy Of Formation Reaction.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Reaction the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. using the values in the above table of standard enthalpies of formation,. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. 193 rows — standard enthalpy of formation. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. standard enthalpy of combustion is. Standard Enthalpy Of Formation Reaction.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpy of combustion is the enthalpy change when one mole. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Enthalpies of Formation and Reaction PowerPoint Presentation Standard Enthalpy Of Formation Reaction the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. the standard enthalpy. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Formation Reaction standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. . Standard Enthalpy Of Formation Reaction.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Formation Reaction standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. a standard enthalpy of formation. Standard Enthalpy Of Formation Reaction.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation Reaction the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. standard enthalpy of. Standard Enthalpy Of Formation Reaction.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. a standard enthalpy of formation (δh°f) is an enthalpy change for. Standard Enthalpy Of Formation Reaction.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Reaction the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. In chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Reaction the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Reaction In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a. Standard Enthalpy Of Formation Reaction.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpy Of Formation Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows — standard enthalpy of formation. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. using the values in the. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. 193 rows — standard enthalpy of formation. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be. Standard Enthalpy Of Formation Reaction.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Reaction a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. a standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. standard enthalpy of combustion is the enthalpy change when one mole of. Standard Enthalpy Of Formation Reaction.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. a standard enthalpy. Standard Enthalpy Of Formation Reaction.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Reaction 193 rows — standard enthalpy of formation. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Standard Enthalpy Of Formation Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. . Standard Enthalpy Of Formation Reaction.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. standard enthalpy of combustion is the enthalpy change when one mole of an. Standard Enthalpy Of Formation Reaction.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation Reaction.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Reaction using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. a standard enthalpy of formation (δh°f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat. Standard Enthalpy Of Formation Reaction.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Reaction the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. standard enthalpy of combustion is the enthalpy change when one mole of an organic compound reacts with molecular. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. the standard enthalpy of reaction. Standard Enthalpy Of Formation Reaction.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Reaction the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the. Standard Enthalpy Of Formation Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. . Standard Enthalpy Of Formation Reaction.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation, also known as the heat of formation, is. Standard Enthalpy Of Formation Reaction.