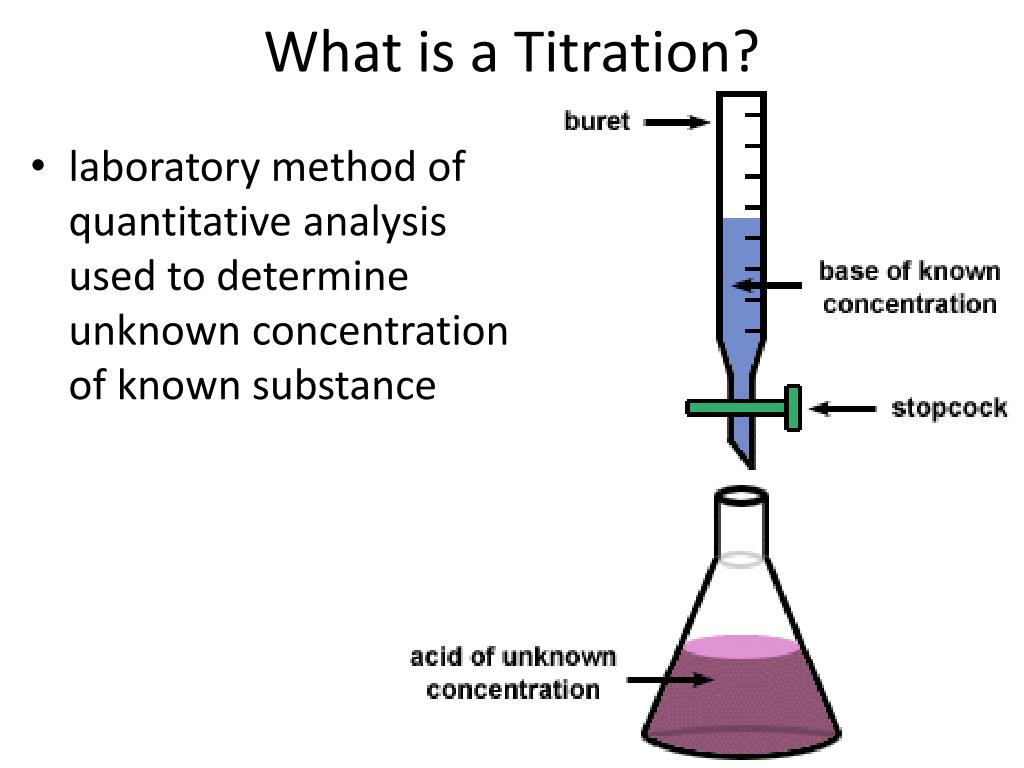
Define Titration With Example . titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What are titrant and analyte. Learn the titration graph and titration equation. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What is the equivalence point of a titration. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be.
from www.slideserve.com
What are titrant and analyte. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Learn the titration graph and titration equation. What is the equivalence point of a titration.
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345
Define Titration With Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What are titrant and analyte. Learn the titration graph and titration equation.
From studylib.net
Experiment 10 Acid Base Titration Definitions Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What are titrant and analyte. titration is the slow addition of. Define Titration With Example.
From www.youtube.com
Chemistry & Biology Define the Term Titration YouTube Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What are titrant and analyte. What is the equivalence point of a titration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition. Define Titration With Example.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Define Titration With Example What are titrant and analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What is the equivalence point of a. Define Titration With Example.
From www.slideshare.net
Complexometric titrations Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What is the equivalence point of a titration. What are titrant. Define Titration With Example.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Define Titration With Example Learn the titration graph and titration equation. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What is the equivalence point. Define Titration With Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the process in which one solution is added to another solution such that. Define Titration With Example.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another. Define Titration With Example.
From theedge.com.hk
Chemistry How To Titration The Edge Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. What are titrant and analyte. What is the equivalence point of a titration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition. Define Titration With Example.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of. Define Titration With Example.
From www.science-revision.co.uk
Titrations Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. Learn the titration graph and titration equation. What are titrant and analyte. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an.. Define Titration With Example.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Define Titration With Example What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What. Define Titration With Example.
From slideplayer.com
U2 S3 L2 Titration page 603 AcidBase Titration pages Titration Step by Step page 838 Define Titration With Example What are titrant and analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What is the equivalence point of a titration. Learn the titration graph and titration equation. titration is the slow addition of one solution of a known concentration. Define Titration With Example.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Education Define Titration With Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What are titrant and analyte. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What is the equivalence point of a titration. titration,. Define Titration With Example.
From www.slideserve.com
PPT Precipitation Titration PowerPoint Presentation ID3571749 Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration (also known as titrimetry[1] and volumetric analysis) is a common. Define Titration With Example.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Define Titration With Example What are titrant and analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. Learn the titration graph and titration equation. What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to. Define Titration With Example.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Learn the titration graph and titration equation. What. Define Titration With Example.
From byjus.com
What is Titration? What are its types? Explain briefly Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Learn the titration graph and titration equation. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What are titrant and. Define Titration With Example.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry Define Titration With Example Learn the titration graph and titration equation. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. . Define Titration With Example.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What are titrant and analyte. titration,. Define Titration With Example.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Define Titration With Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of. Define Titration With Example.
From slideplayer.com
Outline Overview Terminology and Definitions Basic Concept ppt download Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. Learn the titration graph and titration equation. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What are titrant and analyte. What is the equivalence. Define Titration With Example.
From labthink.netlify.app
What is titration and how does it work Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn the titration graph and titration equation. titration, process of chemical analysis in which. Define Titration With Example.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax General Chemistry Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration, process of chemical analysis in which the quantity of some. Define Titration With Example.
From www.youtube.com
Titration Definition, steps and formula YouTube Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What are titrant and analyte. titration (also known as. Define Titration With Example.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID2144898 Define Titration With Example What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What are titrant and analyte. Learn the titration graph and titration equation. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative. Define Titration With Example.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145156 Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration. Learn the titration graph and titration equation. titration refers to a process where the use of a solution of known concentration takes place for the determination of the. Define Titration With Example.
From exoordqpq.blob.core.windows.net
Titrate Meaning Chemistry at Elfriede Downey blog Define Titration With Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Learn the titration graph and titration equation. titration. Define Titration With Example.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Define Titration With Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What are titrant and analyte. titration, process. Define Titration With Example.
From www.thoughtco.com
Titration Definition (Chemistry) Define Titration With Example Learn the titration graph and titration equation. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What are titrant and analyte. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an.. Define Titration With Example.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Foundation Define Titration With Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Learn the titration. Define Titration With Example.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and types of titration YouTube Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. What are titrant and analyte. Learn the titration graph and titration equation.. Define Titration With Example.
From exomtwwgj.blob.core.windows.net
Define Titration Of Strong Acid at Ryan Kardos blog Define Titration With Example What is the equivalence point of a titration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What are titrant and analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.. Define Titration With Example.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345 Define Titration With Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration. Learn the titration graph and titration equation. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. What. Define Titration With Example.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school chemistry Define Titration With Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Learn the titration graph and titration equation. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in. Define Titration With Example.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Define Titration With Example What are titrant and analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to. What is the equivalence point of a titration.. Define Titration With Example.