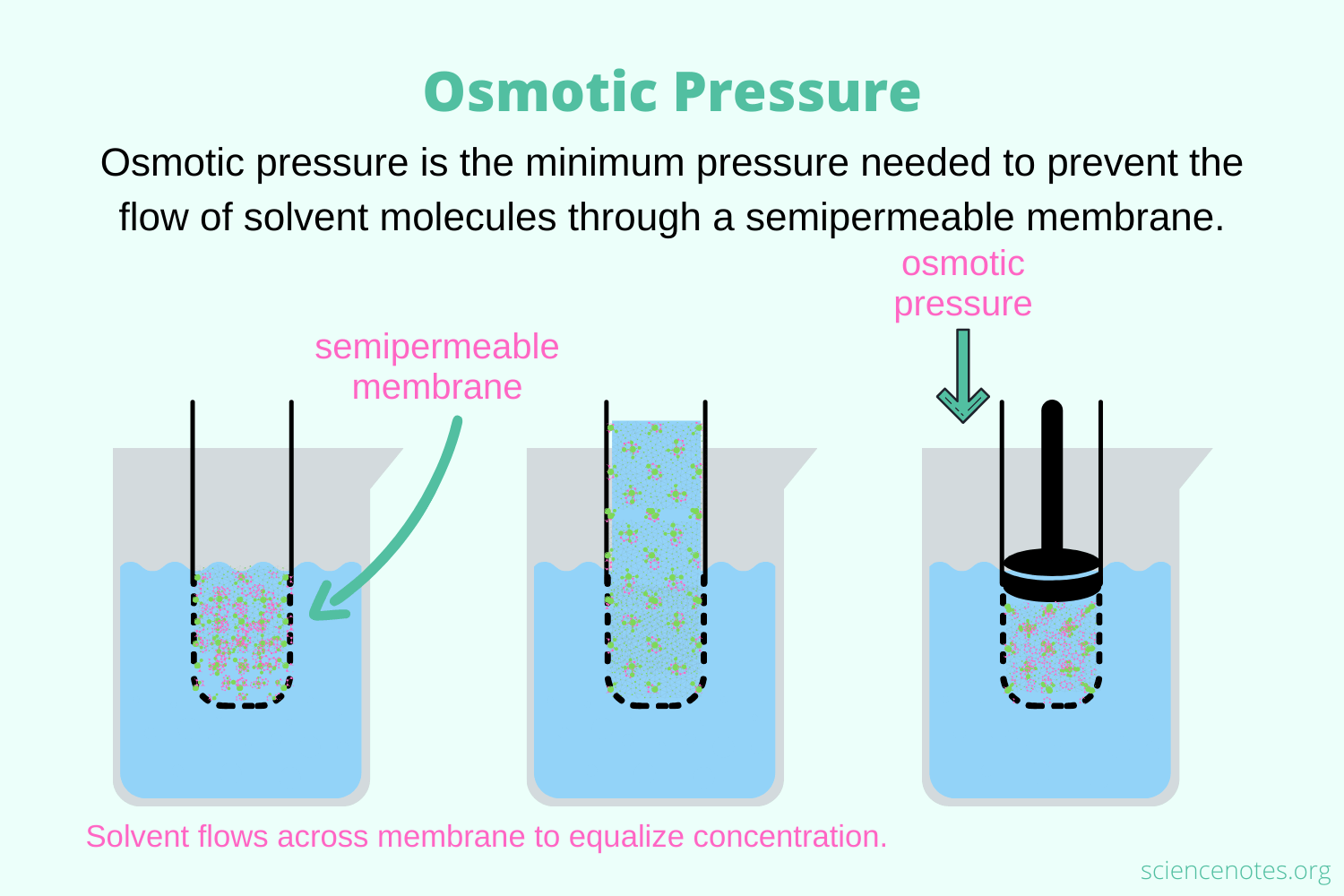
Osmolarity Osmotic Pressure . osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure obeys a law that resembles the ideal gas equation: \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. It is directly proportional to the number of. osmotic pressure is influenced by the concentration of solutes in a solution. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between.
from inspiritvr.com
osmotic pressure obeys a law that resembles the ideal gas equation: osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmotic pressure is influenced by the concentration of solutes in a solution. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. It is directly proportional to the number of.
Osmotic Pressure Study Guide Inspirit Learning Inc
Osmolarity Osmotic Pressure osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure is influenced by the concentration of solutes in a solution. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmotic pressure obeys a law that resembles the ideal gas equation: osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. It is directly proportional to the number of. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where.
From dokumen.tips
(PPT) Water Transport Osmosis, osmolarity, and osmotic pressure DOKUMEN.TIPS Osmolarity Osmotic Pressure osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmotic pressure is influenced by the concentration of solutes in a solution. the maintenance of fluid homeostasis in. Osmolarity Osmotic Pressure.
From drawittoknowit.com
Anatomy & Physiology Fundamentals Osmosis and Osmolarity ditki medical & biological sciences Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. It is directly proportional to the number of. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. . Osmolarity Osmotic Pressure.
From dxozmlqmj.blob.core.windows.net
Osmolality Osmolarity And Tonicity at Eugene Castillo blog Osmolarity Osmotic Pressure osmotic pressure obeys a law that resembles the ideal gas equation: It is directly proportional to the number of. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Importance of Osmosis and Osmotic Pressure PowerPoint Presentation ID9192908 Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. It is directly proportional to the number of. osmolality and, subsequently, osmotic pressure are not affected by the size or charge. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Primary Active Transport PowerPoint Presentation, free download ID2201574 Osmolarity Osmotic Pressure osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmotic pressure is influenced by the concentration of solutes in a solution. osmotic pressure obeys a law that. Osmolarity Osmotic Pressure.
From www.youtube.com
Moles, equivalents, normality, osmoles, osmolarity and osmotic pressure calculation YouTube Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. It is directly proportional to the number of. osmotic pressure obeys a law that resembles the ideal gas equation: . Osmolarity Osmotic Pressure.
From www.studocu.com
Osmolarity, osmotic pressure, p H Osmolarity, osmotic pressure, pH Measuring solute Osmolarity Osmotic Pressure osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. It is directly proportional to the number of. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure obeys a law that resembles the ideal gas equation: \ [ \pi=\dfrac {nrt}. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Chapter 6 Solutions and Colloids PowerPoint Presentation, free download ID5743281 Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmolality (or. Osmolarity Osmotic Pressure.
From chem.libretexts.org
13.7 Osmotic Pressure Chemistry LibreTexts Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure is influenced by the concentration of solutes in a solution. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmotic pressure. Osmolarity Osmotic Pressure.
From drawittoknowit.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis Draw It to Know It Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmolality (or osmolarity) should be used instead of osmotic pressure. Osmolarity Osmotic Pressure.
From www.thoughtco.com
Osmotic Pressure and Tonicity Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure is influenced by the concentration of solutes in a solution. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. . Osmolarity Osmotic Pressure.
From quizgrouchiest.z4.web.core.windows.net
How Does Temperature Affect Osmosis Osmolarity Osmotic Pressure \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure obeys a law that resembles the ideal gas equation: osmolality (or osmolarity). Osmolarity Osmotic Pressure.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmolarity Osmotic Pressure It is directly proportional to the number of. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmolality and, subsequently, osmotic pressure are not affected by. Osmolarity Osmotic Pressure.
From www.youtube.com
GENERAL PHYSIOLOGY MOLE, OSMOLARITY/OSMOLALITY , OSMOTIC PRESSURE // QUICK REVISION YouTube Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure is influenced by the concentration of solutes in a solution. It is directly proportional to the number of. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. . Osmolarity Osmotic Pressure.
From inspiritvr.com
Osmotic Pressure Study Guide Inspirit Learning Inc Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmolarity (osmol) is a way of reporting the total number of particles in a solution. Osmolarity Osmotic Pressure.
From www.youtube.com
osmosis, osmotic and hyrostatic pressure, osmolality vs osmolarity YouTube Osmolarity Osmotic Pressure osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmotic pressure is influenced by the concentration of solutes in a solution. osmotic pressure. Osmolarity Osmotic Pressure.
From pediaa.com
Difference Between Osmotic Pressure and Oncotic Pressure Osmolarity Osmotic Pressure osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine. Osmolarity Osmotic Pressure.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure is influenced by the concentration of solutes in a solution. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmolarity (osmol) is a way of reporting the total number. Osmolarity Osmotic Pressure.
From www.docsity.com
Osmotic pressure and osmolarity Lecture notes Chemistry Docsity Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. \ [ \pi=\dfrac {nrt} {v}=mrt. Osmolarity Osmotic Pressure.
From www.youtube.com
Guyton chapter 4 Osmosis summary osmotic pressure Osmolality Osmolarity Lecture 27 Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure obeys a law that resembles the ideal gas equation: osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmolality and, subsequently, osmotic pressure are not affected by the size or charge. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Tonicity, Osmoticity , Osmolarity , & Osmolality PowerPoint Presentation ID2151960 Osmolarity Osmotic Pressure osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. It is directly proportional to the number of. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmolality (or osmolarity) should be used instead. Osmolarity Osmotic Pressure.
From www.pinterest.com
Vant Hoff formula For calculation of Osmotic pressure ( Note Maximum contribution of Osmolarity Osmotic Pressure osmotic pressure obeys a law that resembles the ideal gas equation: osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. It is directly proportional to the number of. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID4769466 Osmolarity Osmotic Pressure osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine. Osmolarity Osmotic Pressure.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation with Examples Osmolarity Osmotic Pressure osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of. Osmolarity Osmotic Pressure.
From preparatorychemistry.com
Osmosis Osmolarity Osmotic Pressure osmotic pressure obeys a law that resembles the ideal gas equation: the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure.. Osmolarity Osmotic Pressure.
From www.youtube.com
Renal system 13 Osmolarity How to calculate osmotic pressure Van't Hoff law YouTube Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure is influenced by the concentration of solutes in a solution. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. . Osmolarity Osmotic Pressure.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmotic pressure is influenced by the concentration of solutes in a solution. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. . Osmolarity Osmotic Pressure.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation with Examples Osmolarity Osmotic Pressure osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmolality (or osmolarity) should be used instead. Osmolarity Osmotic Pressure.
From dxozmlqmj.blob.core.windows.net
Osmolality Osmolarity And Tonicity at Eugene Castillo blog Osmolarity Osmotic Pressure \ [ \pi=\dfrac {nrt} {v}=mrt \label {eq1}\] where. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality and, subsequently, osmotic pressure are not affected by the size or charge of the solutes but only by the number of solutes. osmotic pressure is influenced by the concentration. Osmolarity Osmotic Pressure.
From www.youtube.com
Osmosis Osmolarity Osmotic Equilibrium Transport Across the Cell Membrane Cell Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmotic pressure is. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT FLUIDS AND ELECTROLYTES PowerPoint Presentation, free download ID3379069 Osmolarity Osmotic Pressure the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. It is directly proportional. Osmolarity Osmotic Pressure.
From slideplayer.com
Chapter 3 Cells The Living Units G.R. Pitts, Ph.D, J.R. Schiller, Ph.D. & James F. Thompson, Ph Osmolarity Osmotic Pressure osmotic pressure is influenced by the concentration of solutes in a solution. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. osmotic pressure obeys a law that resembles the ideal gas equation: the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of. Osmolarity Osmotic Pressure.
From www.slideserve.com
PPT Importance of Osmosis and Osmotic Pressure PowerPoint Presentation ID5749506 Osmolarity Osmotic Pressure the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. It is directly proportional to the number of. osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the. Osmolarity Osmotic Pressure.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series YouTube Osmolarity Osmotic Pressure osmotic pressure depends strongly on the solution properties and has been involved in numerous fundamental. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. the maintenance of fluid homeostasis in each of these compartments is dependent on the excretion of fluids and the. osmotic pressure obeys. Osmolarity Osmotic Pressure.
From droualb.faculty.mjc.edu
Chapter 4 Osmolarity Osmotic Pressure It is directly proportional to the number of. the osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmotic pressure depends strongly on the solution properties and has been involved in. Osmolarity Osmotic Pressure.