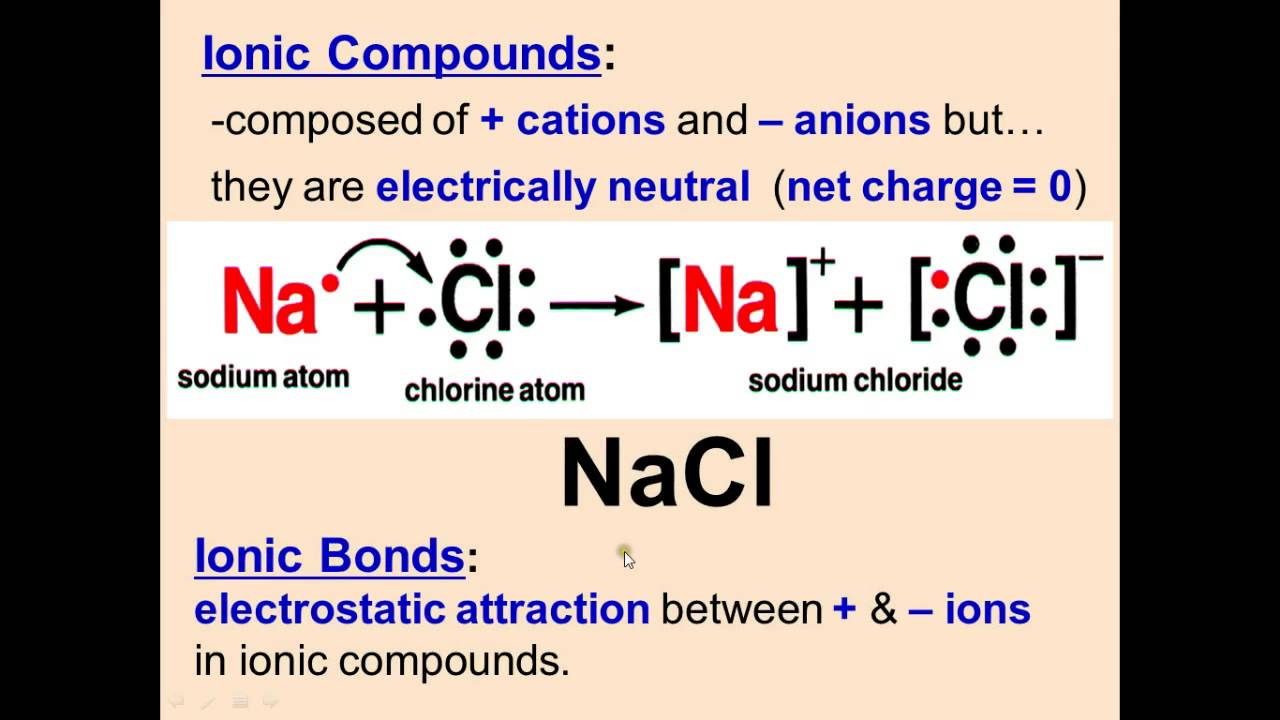
Formula Units . Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. The formula mass is the sum of the atomic. It is not the same as a molecule, which. If you have the number of. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn the difference between a formula unit and a molecule in chemistry. Learn how to identify and write formula units for sodium chloride and calcium. See examples, conventions, and methods for crossing charges to obtain subscripts. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the simplest ratio of ions in an ionic compound.
from
A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the simplest ratio of ions in an ionic compound. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. If you have the number of. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. It is not the same as a molecule, which. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn the difference between a formula unit and a molecule in chemistry.
Formula Units It is not the same as a molecule, which. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. If you have the number of. A formula unit is the simplest ratio of ions in an ionic compound. See examples, conventions, and methods for crossing charges to obtain subscripts. It is not the same as a molecule, which. Learn how to identify and write formula units for sodium chloride and calcium. Learn the difference between a formula unit and a molecule in chemistry. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. The formula mass is the sum of the atomic. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole.
From
Formula Units Learn how to identify and write formula units for sodium chloride and calcium. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the simplest ratio of ions in an ionic compound. It is not the same as a molecule, which. See examples, conventions, and methods for crossing charges to. Formula Units.
From www.chegg.com
Solved How many formula units are contained in 63.0 g of Formula Units A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the simplest ratio of ions in an ionic compound. Learn how to identify and write formula units for sodium chloride and calcium. Learn the difference between a formula unit and a molecule in chemistry. See examples, conventions, and methods for. Formula Units.
From
Formula Units Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. The formula mass is the sum of the atomic. A formula unit shows. Formula Units.
From
Formula Units A formula unit is the simplest ratio of ions in an ionic compound. If you have the number of. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. It is not the same as a molecule, which. A formula unit is the chemical formula of an ionic compound that lists. Formula Units.
From
Formula Units See examples, conventions, and methods for crossing charges to obtain subscripts. It is not the same as a molecule, which. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. The formula mass is the sum of the atomic. A formula unit is the simplest formula of an ionic compound, such as. Formula Units.
From
Formula Units See examples, conventions, and methods for crossing charges to obtain subscripts. If you have the number of. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn the difference between a formula unit and a molecule in chemistry. A formula unit is the simplest formula of an ionic compound, such. Formula Units.
From
Formula Units To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn how to identify and write formula units for sodium chloride and calcium. A formula unit is the simplest ratio of ions in an ionic compound. Learn how to calculate the formula mass of an ionic compound by adding the masses. Formula Units.
From
Formula Units To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. The formula mass is the sum of the atomic. A formula unit is the simplest ratio of ions in an ionic compound. It is not the same as a molecule, which. Learn how to write the chemical formula for a simple. Formula Units.
From
Formula Units Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. See examples, conventions, and methods for crossing charges to obtain subscripts. Learn the difference between a formula unit and a molecule. Formula Units.
From
Formula Units The formula mass is the sum of the atomic. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to write the chemical formula for a simple ionic compound, which is. Formula Units.
From
Formula Units Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. It is not the same as a molecule, which. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the chemical formula of an ionic compound that. Formula Units.
From www.youtube.com
Interpreting units in formulas Mathematics I High School Math Formula Units A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. If you have the number of. See examples, conventions, and methods for crossing charges to obtain subscripts. A formula. Formula Units.
From
Formula Units A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units. Formula Units.
From
Formula Units Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the. Formula Units.
From
Formula Units Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. It is not the same as a molecule, which. See examples, conventions, and methods for crossing charges to obtain. Formula Units.
From
Formula Units It is not the same as a molecule, which. The formula mass is the sum of the atomic. A formula unit is the simplest ratio of ions in an ionic compound. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. Learn how to identify. Formula Units.
From rileyoiblair.blogspot.com
Determination of the Formula Unit of a Compound RileyoiBlair Formula Units See examples, conventions, and methods for crossing charges to obtain subscripts. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. It is not the same as a molecule, which. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the. Formula Units.
From
Formula Units The formula mass is the sum of the atomic. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. If you have the number of. Learn the difference between a formula unit and a molecule in chemistry. To convert formula units to moles, you use. Formula Units.
From slidesharetrick.blogspot.com
What Is A Formula Unit slidesharetrick Formula Units Learn the difference between a formula unit and a molecule in chemistry. If you have the number of. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn. Formula Units.
From
Formula Units A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio. Formula Units.
From
Formula Units The formula mass is the sum of the atomic. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. Learn the difference between a formula. Formula Units.
From studylib.net
Formula Formula Units To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. A formula unit is the simplest ratio of ions in an ionic compound. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. A formula unit is the simplest formula of an ionic. Formula Units.
From
Formula Units Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. It is not the same as a molecule, which. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. The formula mass is. Formula Units.
From
Formula Units If you have the number of. The formula mass is the sum of the atomic. A formula unit is the simplest ratio of ions in an ionic compound. See examples, conventions, and methods for crossing charges to obtain subscripts. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. A formula. Formula Units.
From www.slideshare.net
Chem unit 6 presentation Formula Units The formula mass is the sum of the atomic. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge. Learn how to write the chemical formula. Formula Units.
From
Formula Units A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. If you have the number of. A formula unit is the simplest ratio of ions in an ionic compound. Learn how to identify and write formula. Formula Units.
From www.pinterest.com
SI Unit Conversion Cheat Sheet MCAT Cheat Sheet Chemistry education Formula Units Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. The formula mass is the sum of the atomic. A formula unit is the simplest ratio of ions in. Formula Units.
From
Formula Units It is not the same as a molecule, which. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. Learn how to write the chemical formula for a simple ionic compound, which is the minimum proportion of ions in the crystal lattice. Learn the difference between a formula unit and a molecule in chemistry.. Formula Units.
From www.coursehero.com
[Solved] How do I calculate the formula units of a compound given its Formula Units The formula mass is the sum of the atomic. See examples, conventions, and methods for crossing charges to obtain subscripts. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. If you have the number of. A formula unit is the chemical formula of an ionic compound that. Formula Units.
From
Formula Units A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. Learn how to calculate the formula mass of an ionic compound by adding the masses of each individual atom in the formula. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. It. Formula Units.
From mavink.com
What Is Formula Unit Formula Units It is not the same as a molecule, which. If you have the number of. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the simplest ratio of ions in an ionic compound. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula. Formula Units.
From
Formula Units A formula unit is the simplest ratio of ions in an ionic compound. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn how to calculate the formula mass of an ionic compound by. Formula Units.
From mavink.com
What Is Formula Unit Formula Units A formula unit shows the ratio of ions in an ionic compound, while a molecule is the smallest. A formula unit is the simplest ratio of ions in an ionic compound. It is not the same as a molecule, which. If you have the number of. Learn how to write the chemical formula for a simple ionic compound, which is. Formula Units.
From
Formula Units The formula mass is the sum of the atomic. Learn how to identify and write formula units for sodium chloride and calcium. It is not the same as a molecule, which. A formula unit is the simplest formula of an ionic compound, such as nacl or mgcl2. A formula unit is the simplest ratio of ions in an ionic compound.. Formula Units.
From
Formula Units See examples, conventions, and methods for crossing charges to obtain subscripts. The formula mass is the sum of the atomic. If you have the number of. To convert formula units to moles, you use avogadro's number, which is 6.022 x 1023 formula units per mole. Learn how to calculate the formula mass of an ionic compound by adding the masses. Formula Units.