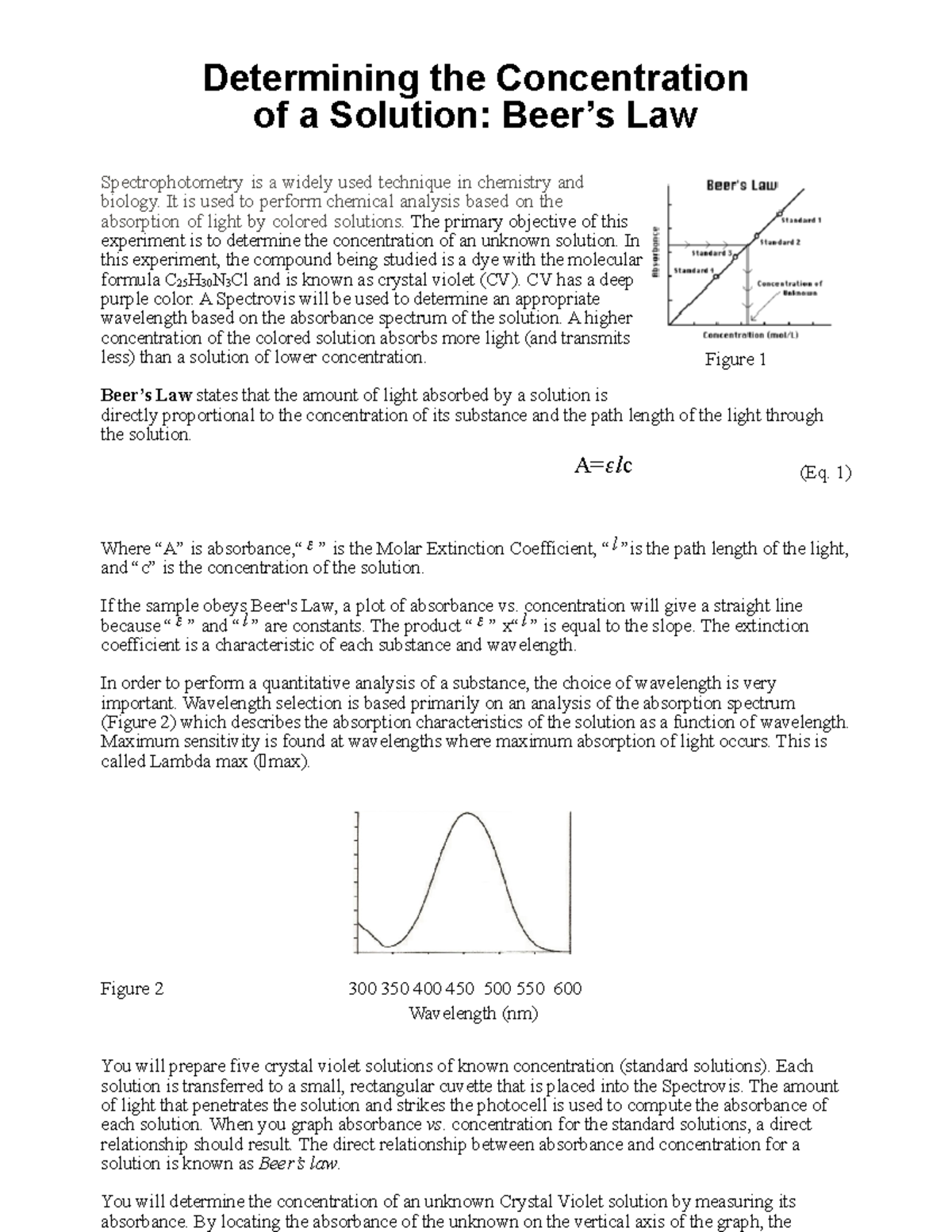
How Does Beer's Law Work . In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The premise is that a light beam becomes weaker as.
from www.studocu.com
Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Spectrophotometry involves passing a beam of ultraviolet. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance.
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the
How Does Beer's Law Work Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are. How Does Beer's Law Work.
From studylib.net
Beer`s Law How Does Beer's Law Work The premise is that a light beam becomes weaker as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that. How Does Beer's Law Work.
From www.thoughtco.com
Beer's Law Definition and Equation How Does Beer's Law Work In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. Since. How Does Beer's Law Work.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint How Does Beer's Law Work Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. The premise is that a light beam becomes weaker as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional. How Does Beer's Law Work.
From www.youtube.com
Beer's Law Explanation and Example YouTube How Does Beer's Law Work Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that. How Does Beer's Law Work.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations How Does Beer's Law Work The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily finds applications in the field of spectrophotometry. How Does Beer's Law Work.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship. How Does Beer's Law Work.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation How Does Beer's Law Work The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the. How Does Beer's Law Work.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the. How Does Beer's Law Work.
From www.slideserve.com
PPT Principles of Spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Spectrophotometry involves passing a beam of ultraviolet. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The. How Does Beer's Law Work.
From www.youtube.com
Lambert Beer Law Absorption laws spectroscopy spectroscopy organic How Does Beer's Law Work The premise is that a light beam becomes weaker as. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional. How Does Beer's Law Work.
From www.youtube.com
Beer's Law Overview YouTube How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly. How Does Beer's Law Work.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law How Does Beer's Law Work Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The premise is that a light beam becomes weaker as. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In most experiments, molar absorptivity (ε) and the. How Does Beer's Law Work.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts. How Does Beer's Law Work.
From sciencenotes.org
Beer's Law Equation and Example How Does Beer's Law Work The premise is that a light beam becomes weaker as. Spectrophotometry involves passing a beam of ultraviolet. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Since the concentration, path length and molar absorptivity are all directly proportional. How Does Beer's Law Work.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples How Does Beer's Law Work The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily finds applications in the field of spectrophotometry. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation. How Does Beer's Law Work.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily. How Does Beer's Law Work.
From www.slideserve.com
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer How Does Beer's Law Work In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Spectrophotometry involves passing a beam of ultraviolet. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lamberts law states a relationship. How Does Beer's Law Work.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID795528 How Does Beer's Law Work Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave. How Does Beer's Law Work.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID2225328 How Does Beer's Law Work Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the. How Does Beer's Law Work.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء How Does Beer's Law Work The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that. How Does Beer's Law Work.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. How Does Beer's Law Work.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Spectrophotometry involves passing a beam of ultraviolet. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The. How Does Beer's Law Work.
From www.youtube.com
Derivation of Beer Lambert Law YouTube How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise. How Does Beer's Law Work.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW How Does Beer's Law Work In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lamberts law states a relationship between the. How Does Beer's Law Work.
From www.youtube.com
Ch 17 7 Beer's Law is Additive for All Components YouTube How Does Beer's Law Work Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Spectrophotometry involves passing a beam of ultraviolet. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore,. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. The premise is that a light beam becomes weaker. How Does Beer's Law Work.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example How Does Beer's Law Work Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b). How Does Beer's Law Work.
From www.thoughtco.com
Beer's Law Definition and Equation How Does Beer's Law Work Spectrophotometry involves passing a beam of ultraviolet. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties. How Does Beer's Law Work.
From www.scribd.com
Lambert Beer Law History Absorbance Radiation How Does Beer's Law Work Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Spectrophotometry involves passing a beam of ultraviolet. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light. How Does Beer's Law Work.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Does Beer's Law Work The premise is that a light beam becomes weaker as. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly proportional to its light. How Does Beer's Law Work.