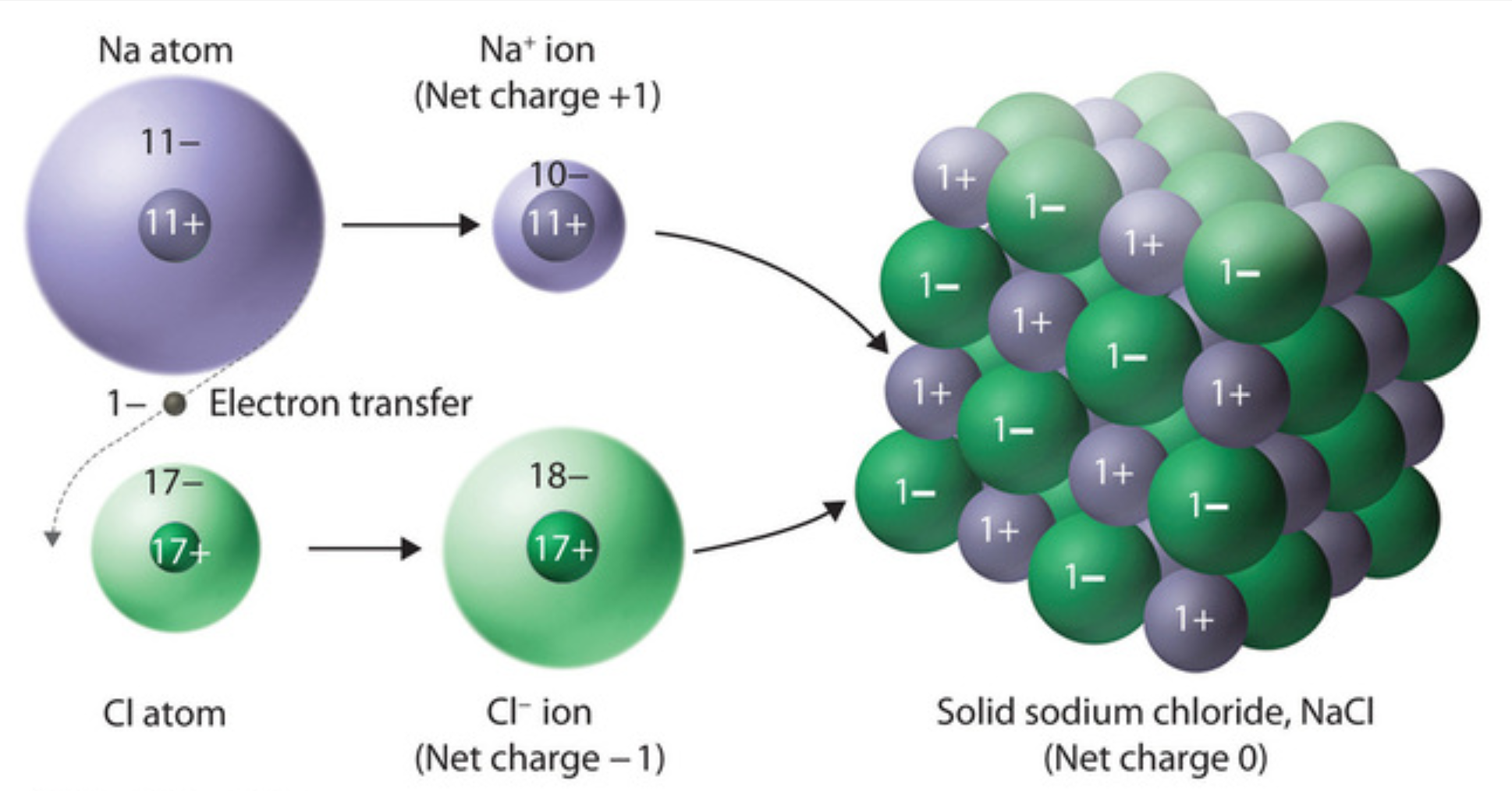
Chlorine Table Salt Equation . na + cl 2 → 2nacl. the formula for table salt is nacl. The arrangement forms a regular octahedron. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the molecular formula of table salt—sodium chloride—is nacl. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride.
from chem.libretexts.org
sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. the molecular formula of table salt—sodium chloride—is nacl. na + cl 2 → 2nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. The arrangement forms a regular octahedron. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. the formula for table salt is nacl. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula.
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts
Chlorine Table Salt Equation Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. the formula for table salt is nacl. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. the molecular formula of table salt—sodium chloride—is nacl. na + cl 2 → 2nacl. The arrangement forms a regular octahedron.
From www.numerade.com
SOLVED Question 9 solid sodium chloride is produced by the reaction of chlorine gas and solid Chlorine Table Salt Equation sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the formula for table salt is nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the synthesis reaction that produces table salt,. Chlorine Table Salt Equation.
From cartoondealer.com
Sodium Chloride Rock Salt, Halite, Table Salt, Chemical Structure. Vector Illustration Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. the formula for table salt is nacl. In the solid lattice, each ion is surrounded. Chlorine Table Salt Equation.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula. Stock Vector Adobe Stock Chlorine Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. The arrangement forms a regular octahedron. the formula for table salt is nacl. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes. Chlorine Table Salt Equation.
From stock.adobe.com
Structural chemical formula of sodium chloride molecule on a black chalkboard with spoonful of Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ. Chlorine Table Salt Equation.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Chlorine Table Salt Equation the formula for table salt is nacl. The arrangement forms a regular octahedron. na + cl 2 → 2nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8]. Chlorine Table Salt Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Table Salt Equation sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. the synthesis reaction that produces table salt, or sodium. Chlorine Table Salt Equation.
From proper-cooking.info
Chemical Formula For Sodium Chloride Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. na + cl 2 → 2nacl. the formula for table salt is nacl. the synthesis reaction that produces table salt, or. Chlorine Table Salt Equation.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Chlorine Table Salt Equation Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the formula for table salt is nacl. The arrangement forms a regular octahedron. sodium chloride / ˌ s oʊ d. Chlorine Table Salt Equation.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Table Salt Equation The arrangement forms a regular octahedron. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. the. Chlorine Table Salt Equation.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the synthesis reaction that produces table. Chlorine Table Salt Equation.
From exomdamui.blob.core.windows.net
Table Salt Atomic Formula at Pete Alvarez blog Chlorine Table Salt Equation sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination. Chlorine Table Salt Equation.
From exoilfotb.blob.core.windows.net
What Is Table Salt In Chemistry at Christina Stotts blog Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. the formula for table salt is nacl. na + cl 2 → 2nacl. the molecular formula of table salt—sodium chloride—is nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine.. Chlorine Table Salt Equation.
From www.saubhaya.com
Table Salt Chemical Makeup Saubhaya Makeup Chlorine Table Salt Equation sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. the formula for table salt is nacl. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions. Chlorine Table Salt Equation.
From www.chegg.com
Solved +3 The structure of a sodium chloride (table salt) Chlorine Table Salt Equation na + cl 2 → 2nacl. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. the molecular formula of table salt—sodium chloride—is nacl. the formula for table salt. Chlorine Table Salt Equation.
From exoxnecug.blob.core.windows.net
Table Salt Sodium Chloride Elements at Francisco White blog Chlorine Table Salt Equation the formula for table salt is nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the molecular formula of table salt—sodium chloride—is nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and. Chlorine Table Salt Equation.
From www.youtube.com
Electrolysis of Sodium Chloride Electrochemistry YouTube Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. The arrangement forms a regular octahedron.. Chlorine Table Salt Equation.
From www.istockphoto.com
Vector Ballandstick Model Of Chemical Substance Icon Of Sodium Chloride Molecule Commonly Known Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. the molecular formula of table salt—sodium chloride—is nacl. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. The arrangement forms. Chlorine Table Salt Equation.
From riversfansite.blogspot.com
Table Salt Chemical Formula Sodium chloride , commonly known as salt (although sea salt also Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. In the solid lattice,. Chlorine Table Salt Equation.
From www.chegg.com
Solved 2. Table salt has a molecular formula of NaCl (i.e. 1 Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. na + cl 2 → 2nacl. sodium chloride, also known as table salt, is an ionic compound with. Chlorine Table Salt Equation.
From www.thoughtco.com
Table Salt Molecular Formula Sodium Chloride Chlorine Table Salt Equation The arrangement forms a regular octahedron. the molecular formula of table salt—sodium chloride—is nacl. na + cl 2 → 2nacl. the formula for table salt is nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. sodium chloride, also known as. Chlorine Table Salt Equation.
From www.animalia-life.club
Table Salt Chemical Structure Chlorine Table Salt Equation the formula for table salt is nacl. the molecular formula of table salt—sodium chloride—is nacl. na + cl 2 → 2nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. Two moles of chlorine gas reacts with one mole of sodium to. Chlorine Table Salt Equation.
From www.bharatagritech.com
Sodium Chloride (table Salt), Chemical Skeletal Formula, 44 OFF Chlorine Table Salt Equation The arrangement forms a regular octahedron. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole. Chlorine Table Salt Equation.
From www.alamy.com
Sodium chloride (table salt), chemical structure. Blue skeletal formula on white background Chlorine Table Salt Equation the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. The arrangement forms a regular octahedron. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is. Chlorine Table Salt Equation.
From www.youtube.com
How to Balance Na + Cl2 = NaCl YouTube Chlorine Table Salt Equation the formula for table salt is nacl. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula.. Chlorine Table Salt Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Table Salt Equation sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly. Chlorine Table Salt Equation.
From www.alamy.com
Sodium chloride (table salt), chemical structure. White skeletal formula on dark teal gradient Chlorine Table Salt Equation The arrangement forms a regular octahedron. the molecular formula of table salt—sodium chloride—is nacl. na + cl 2 → 2nacl. the formula for table salt is nacl. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. Two moles of chlorine gas reacts. Chlorine Table Salt Equation.
From www.youtube.com
How to Draw the Lewis Dot Structure for NaCl Sodium chloride YouTube Chlorine Table Salt Equation the formula for table salt is nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. sodium chloride, also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. nacl = na + cl is a decomposition reaction where one. Chlorine Table Salt Equation.
From gbu-taganskij.ru
Sodium Chloride (table Salt), Chemical Skeletal Formula, 48 OFF Chlorine Table Salt Equation the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of. Chlorine Table Salt Equation.
From www.youtube.com
Structure of NaCl (Sodium chloride) YouTube Chlorine Table Salt Equation Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. The arrangement forms a regular octahedron. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the synthesis reaction that produces table salt, or sodium chloride (nacl),. Chlorine Table Salt Equation.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. na + cl 2 → 2nacl. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. sodium chloride, also known. Chlorine Table Salt Equation.
From www.alamy.com
Sodium chloride structure hires stock photography and images Alamy Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. the synthesis reaction that produces table salt, or sodium chloride. Chlorine Table Salt Equation.
From www.alamy.com
Sodium chloride table salt hires stock photography and images Alamy Chlorine Table Salt Equation the formula for table salt is nacl. the molecular formula of table salt—sodium chloride—is nacl. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride. The arrangement forms a regular octahedron. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8]. Chlorine Table Salt Equation.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Table Salt Equation nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. the formula for table salt is nacl. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. sodium chloride, also known as table salt, is an ionic compound. Chlorine Table Salt Equation.
From www.dreamstime.com
Sodium Chloride or Rock Salt, Halite, Table Salt, Chemical Structure. Stock Vector Chlorine Table Salt Equation In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride [nacl] decomposes into one mole of solid. na + cl 2 → 2nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves. Chlorine Table Salt Equation.
From www.slideserve.com
PPT Making Molecules PowerPoint Presentation, free download ID6226902 Chlorine Table Salt Equation na + cl 2 → 2nacl. sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Two moles of chlorine gas. Chlorine Table Salt Equation.