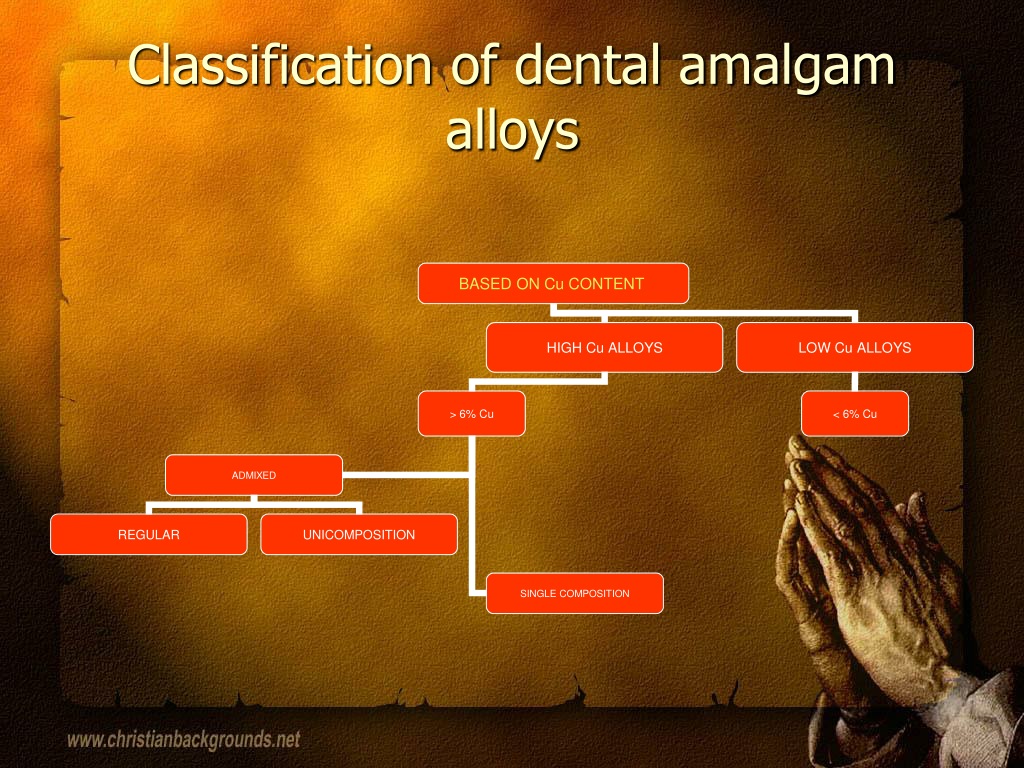
Dental Amalgam Fda Classification . Classification of dental amalgam, reclassification of dental mercury, designation of special controls. In 2009, the fda reclassified dental mercury from class i (lowest risk). 872.3070 dental amalgam, mercury, and amalgam alloy. fda reclassification of dental amalgam. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. on july 28, 2009, fda issued a final rule that: dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Dental amalgam is a device.
from www.slideserve.com
the food and drug administration (fda) has developed this guidance as the special control to support the classification of. In 2009, the fda reclassified dental mercury from class i (lowest risk). dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. fda reclassification of dental amalgam. Dental amalgam is a device. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. on july 28, 2009, fda issued a final rule that: Classification of dental amalgam, reclassification of dental mercury, designation of special controls. 872.3070 dental amalgam, mercury, and amalgam alloy.
PPT AMALGAM PowerPoint Presentation, free download ID9694003
Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. fda reclassification of dental amalgam. Dental amalgam is a device. on july 28, 2009, fda issued a final rule that: the food and drug administration (fda) has developed this guidance as the special control to support the classification of. 872.3070 dental amalgam, mercury, and amalgam alloy. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. In 2009, the fda reclassified dental mercury from class i (lowest risk). Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental.
From www.slideserve.com
PPT Dental Amalgam PowerPoint Presentation ID973867 Dental Amalgam Fda Classification fda reclassification of dental amalgam. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. Dental amalgam is a device. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. In. Dental Amalgam Fda Classification.
From www.studocu.com
14 Amalgam chapter 14 Dental amalgam ( 1 Lecture. ) . .. .. 1 Lecture. Contents of lecture Dental Amalgam Fda Classification on july 28, 2009, fda issued a final rule that: fda reclassification of dental amalgam. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Classification of dental amalgam, reclassification of dental mercury, designation. Dental Amalgam Fda Classification.
From www.fda.gov
Dental Amalgam Fillings Graphics FDA Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). Dental amalgam is a device. fda reclassification of dental amalgam. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. on july 28, 2009, fda issued a final rule that: the food and drug administration (fda) has developed this. Dental Amalgam Fda Classification.
From www.dentistryiq.com
Mustknow classifications of dental caries for the national dental hygiene boards DentistryIQ Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). fda reclassification of dental amalgam. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Dental amalgam is a device. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. dental amalgam is a device that consists of a metallic. Dental Amalgam Fda Classification.
From healthinspireddentistry.com
FDA reveals high risk people of amalgam fillings Mercuryfree Dentist, Cleveland Dental Amalgam Fda Classification Dental amalgam is a device. 872.3070 dental amalgam, mercury, and amalgam alloy. In 2009, the fda reclassified dental mercury from class i (lowest risk). Classification of dental amalgam, reclassification of dental mercury, designation of special controls. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid.. Dental Amalgam Fda Classification.
From www.dentistrytoday.com
FDA Infographics Advise Patients About Amalgam Risks Dentistry Today Dental Amalgam Fda Classification dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Dental amalgam is a device. In 2009, the fda reclassified dental mercury from class i (lowest risk). Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. (1) reclassified mercury from a. Dental Amalgam Fda Classification.
From pocketdentistry.com
12. The Dental Examination Pocket Dentistry Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. (1) reclassified mercury from a class i (least risk) device to class. Dental Amalgam Fda Classification.
From www.pagedental.com
Potential danger of dental amalgam for some individuals FDA Dental Amalgam Fda Classification (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. fda reclassification of dental amalgam. Dental amalgam is a. Dental Amalgam Fda Classification.
From www.fda.gov
Dental Amalgam Fillings FDA Dental Amalgam Fda Classification dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. 872.3070 dental amalgam, mercury, and amalgam alloy. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. on july 28, 2009, fda issued a final. Dental Amalgam Fda Classification.
From www.studypool.com
SOLUTION Dental amalgam ppt 2 dm lectures Studypool Dental Amalgam Fda Classification 872.3070 dental amalgam, mercury, and amalgam alloy. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Dental amalgam is a device. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. on july 28, 2009, fda issued a. Dental Amalgam Fda Classification.
From www.youtube.com
Dental amalgam classification or types YouTube Dental Amalgam Fda Classification (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. on july 28, 2009, fda issued a final rule that: Dental amalgam is a device. In 2009, the fda reclassified dental mercury from class i (lowest risk). Classification of dental amalgam, reclassification of dental mercury, designation of special controls. the food and. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT AMALGAM PowerPoint Presentation, free download ID9694003 Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. fda reclassification of dental amalgam. Dental amalgam is a device. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. the food and drug administration (fda) has developed this guidance as the special control to support the classification. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT Dental Amalgam PowerPoint Presentation, free download ID973867 Dental Amalgam Fda Classification fda reclassification of dental amalgam. In 2009, the fda reclassified dental mercury from class i (lowest risk). Dental amalgam is a device. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper,. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT Dental Amalgam PowerPoint Presentation, free download ID6710902 Dental Amalgam Fda Classification fda reclassification of dental amalgam. Dental amalgam is a device. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. 872.3070 dental amalgam, mercury, and amalgam alloy. (1) reclassified mercury from. Dental Amalgam Fda Classification.
From www.dentistrytoday.com
FDA Expands Dental Amalgam Dentistry Today Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. In 2009, the fda reclassified dental mercury from class i (lowest risk). 872.3070 dental amalgam, mercury, and amalgam alloy. Dental amalgam is a device. fda reclassification of dental amalgam.. Dental Amalgam Fda Classification.
From gamma.app
Classification and Setting Reaction of Dental Amalgam Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). on july 28, 2009, fda issued a final rule that: (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. Dental amalgam is a device. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. fda reclassification of. Dental Amalgam Fda Classification.
From www.totalcaredental.com
Dangers of Dental Amalgam Fillings Total Care Dental Holistic Dentistry Dental Amalgam Fda Classification the food and drug administration (fda) has developed this guidance as the special control to support the classification of. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;.. Dental Amalgam Fda Classification.
From www.youtube.com
Dental Amalgam Part 1 Classification Uses Low Copper Amalgam High Copper Amalgam YouTube Dental Amalgam Fda Classification on july 28, 2009, fda issued a final rule that: (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. 872.3070 dental amalgam, mercury, and amalgam alloy. In 2009, the fda reclassified dental mercury from class i (lowest risk). the food and drug administration (fda) has developed this guidance as the special. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT DENTAL AMALGAM PowerPoint Presentation, free download ID6299866 Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls. on july 28, 2009, fda issued a final rule that: the food and drug administration (fda) has developed this guidance as the special control to support the classification of. 872.3070 dental amalgam, mercury, and amalgam alloy. fda reclassification of dental amalgam. (1) reclassified mercury from. Dental Amalgam Fda Classification.
From exoxelnim.blob.core.windows.net
Dental Amalgam And Composite Materials at Helen Drake blog Dental Amalgam Fda Classification Dental amalgam is a device. fda reclassification of dental amalgam. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for. Dental Amalgam Fda Classification.
From www.studypool.com
SOLUTION Classification of dental amalgam Studypool Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). Classification of dental amalgam, reclassification of dental mercury, designation of special controls. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. fda reclassification of dental amalgam. dental amalgam is a device that consists of a metallic alloy, such as silver, tin,. Dental Amalgam Fda Classification.
From www.fda.gov
Dental Amalgam Fillings Graphics FDA Dental Amalgam Fda Classification Dental amalgam is a device. on july 28, 2009, fda issued a final rule that: the food and drug administration (fda) has developed this guidance as the special control to support the classification of. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid.. Dental Amalgam Fda Classification.
From price-pottenger.org
In The News FDA Issues New Safety Guidelines for Use of MercuryContaining Dental Amalgam Dental Amalgam Fda Classification on july 28, 2009, fda issued a final rule that: dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Classification of dental amalgam, reclassification of dental mercury, designation of special. Dental Amalgam Fda Classification.
From lessonfullpawnticket.z21.web.core.windows.net
Tooth Colors And Charting Dental Amalgam Fda Classification fda reclassification of dental amalgam. In 2009, the fda reclassified dental mercury from class i (lowest risk). dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. on july 28, 2009, fda issued a final rule that: the food and drug administration (fda). Dental Amalgam Fda Classification.
From www.researchgate.net
(PDF) SURVEY ON TYPES OF DENTAL AMALGAM IN MEDICINE Dental Amalgam Fda Classification on july 28, 2009, fda issued a final rule that: Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. 872.3070 dental amalgam, mercury, and amalgam alloy. dental amalgam is a device that consists of a metallic alloy, such as silver, tin,. Dental Amalgam Fda Classification.
From www.studocu.com
Dental amalgam part 1 Restorative Dentistry Is an alloy made by mixing liquid mercury (Hg Dental Amalgam Fda Classification In 2009, the fda reclassified dental mercury from class i (lowest risk). (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. fda reclassification of dental amalgam. 872.3070 dental. Dental Amalgam Fda Classification.
From www.youtube.com
Dental Amalgam 1 Composition & classification YouTube Dental Amalgam Fda Classification (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. on july 28, 2009, fda issued a final rule that: dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is. Dental Amalgam Fda Classification.
From www.pinterest.jp
5. Dental Amalgam Dentistry, Dental restoration, Dental Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. on july 28, 2009, fda issued a final rule that: 872.3070 dental amalgam, mercury, and amalgam alloy. fda reclassification of dental amalgam. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Dental amalgam. Dental Amalgam Fda Classification.
From www.studypool.com
SOLUTION Classification of dental amalgam Studypool Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. Dental amalgam is a device. dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. the food and drug administration (fda) has developed this guidance as the special control to support. Dental Amalgam Fda Classification.
From rumble.com
MERCURY 102 FDA Classification versus Amalgam Risk Assessment Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls. Dental amalgam is a device. fda reclassification of dental amalgam. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. dental amalgam. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT Dental Amalgam PowerPoint Presentation, free download ID3931996 Dental Amalgam Fda Classification Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. fda reclassification of dental amalgam. on july 28, 2009, fda issued a final rule that: In 2009, the fda reclassified dental mercury from class. Dental Amalgam Fda Classification.
From www.amazon.com
Dental Devices Classification of Dental Amalgam, Reclassification of Dental Mercury Dental Amalgam Fda Classification dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Dental amalgam is a device. 872.3070 dental amalgam, mercury, and amalgam alloy. In 2009, the fda. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT Dental Amalgam PowerPoint Presentation, free download ID6710902 Dental Amalgam Fda Classification dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. In 2009, the fda reclassified dental mercury from class i (lowest risk). the food and drug administration (fda) has developed this guidance as the special control to support the classification of. on july 28,. Dental Amalgam Fda Classification.
From www.slideserve.com
PPT KOMPOSISI AMALGAM PowerPoint Presentation, free download ID4290441 Dental Amalgam Fda Classification dental amalgam is a device that consists of a metallic alloy, such as silver, tin, copper, and zinc, that is mixed with liquid. fda reclassification of dental amalgam. (1) reclassified mercury from a class i (least risk) device to class ii (more risk) device;. Dental amalgam is a device. In 2009, the fda reclassified dental mercury from class. Dental Amalgam Fda Classification.
From agreatertown.com
DR. MERCOLA, FDA AMALGAM, SMART CERTIFIED DENTIST' Dental Implant Dentists North Providence, RI Dental Amalgam Fda Classification 872.3070 dental amalgam, mercury, and amalgam alloy. Dental amalgam is a device. the food and drug administration (fda) has developed this guidance as the special control to support the classification of. Classification of dental amalgam, reclassification of dental mercury, designation of special controls. Classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental. fda. Dental Amalgam Fda Classification.