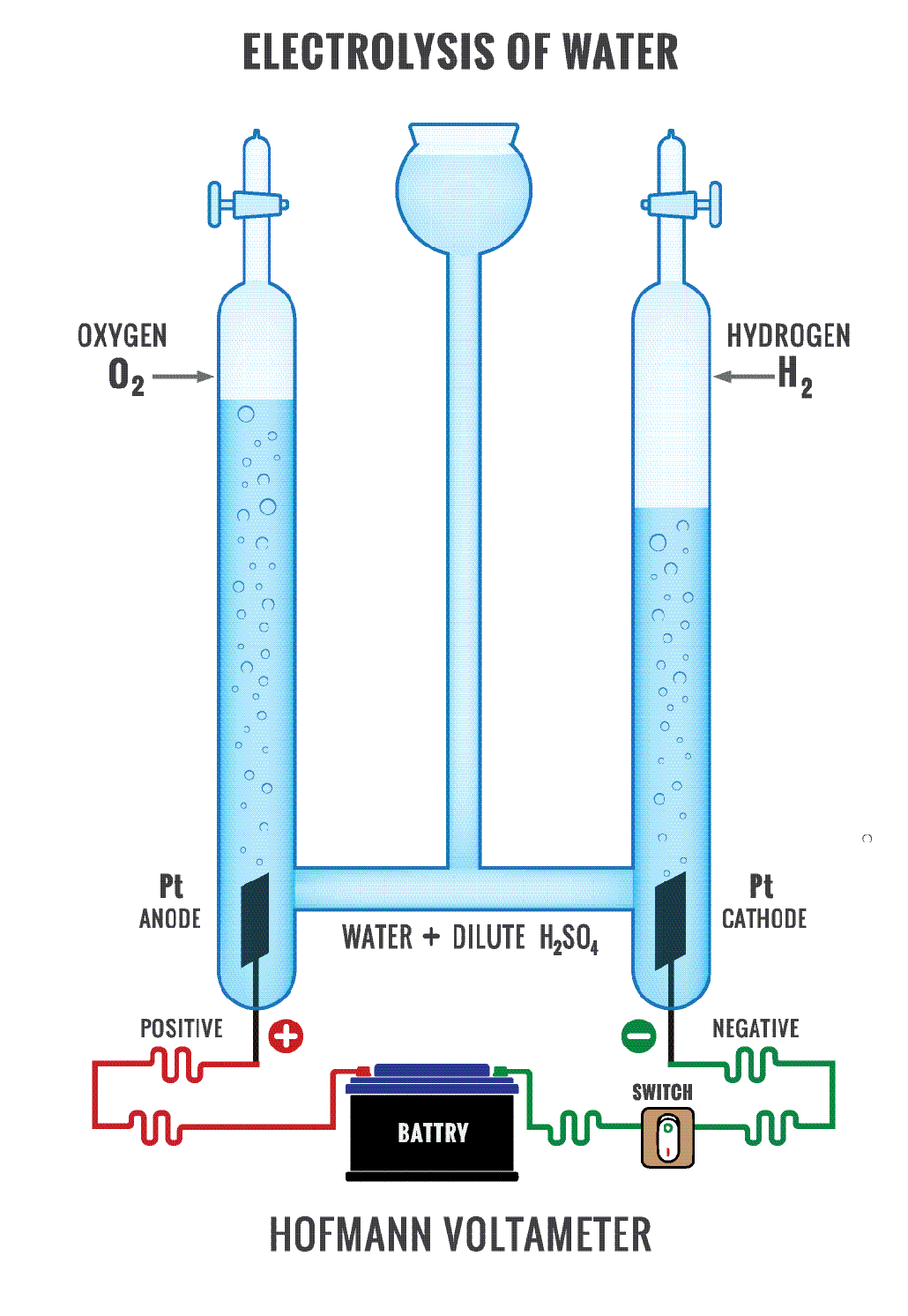
Does Electrolysis Produce Electricity . Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction.
from www.sciencenewsforstudents.org
The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis is the process of using electricity to split water into hydrogen and. An external voltage is applied to drive a nonspontaneous. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or.
Explainer What is an electrode? Science News for Students
Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. Electrolysis is the process of using electricity to split water into hydrogen and. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or.
From socratic.org
Electrolysis Chemistry Socratic Does Electrolysis Produce Electricity The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using electricity to split water into hydrogen and. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. A chemical change takes place. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen. Does Electrolysis Produce Electricity.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: A chemical change takes place when atoms and ions lose or. Electrolysis is the process of using electricity to split water into hydrogen and.. Does Electrolysis Produce Electricity.
From mungfali.com
Electrolysis Process Diagram Does Electrolysis Produce Electricity In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen and. An external voltage is applied to drive a nonspontaneous. For example, electrolysis is a process that involves forcing electricity through. Does Electrolysis Produce Electricity.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis is the process of using electricity to split water into hydrogen and. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. An external voltage is applied to drive a nonspontaneous.. Does Electrolysis Produce Electricity.
From byjus.com
Does Electrolysis produce electricity? Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions. Does Electrolysis Produce Electricity.
From www.energy.dtu.dk
How does electrolysis work Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis is the process of using electricity to split water into hydrogen. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT What is electrolysis? PowerPoint Presentation ID4564902 Does Electrolysis Produce Electricity An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change. Does Electrolysis Produce Electricity.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT Hydrogen electrolysis PowerPoint Presentation, free download ID Does Electrolysis Produce Electricity The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: A chemical change takes place when atoms and ions lose or. For example, electrolysis is a. Does Electrolysis Produce Electricity.
From www.energy.gov
Hydrogen Production Electrolysis Department of Energy Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur.. Does Electrolysis Produce Electricity.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a. Does Electrolysis Produce Electricity.
From chemistryjee.blogspot.com
chemistry world KOLBES ELECTROLYSIS Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. A chemical change takes place when atoms and ions lose or. An external voltage is applied to drive a nonspontaneous. Electrolysis is the. Does Electrolysis Produce Electricity.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing. Does Electrolysis Produce Electricity.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. A chemical change takes place when. Does Electrolysis Produce Electricity.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Does Electrolysis Produce Electricity Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a. Does Electrolysis Produce Electricity.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Does Electrolysis Produce Electricity An external voltage is applied to drive a nonspontaneous. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using electricity to split water into hydrogen and. A chemical change takes place when atoms and ions lose or. In an electrolytic cell, however, the opposite process, called electrolysis,. Does Electrolysis Produce Electricity.
From www.chemicals.co.uk
Why Does Potassium Iodide Solution Conduct Electricity? Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. An external voltage is applied to drive a nonspontaneous. A chemical change takes place when atoms and ions lose or. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The primary mechanism of electrolysis is the interchange. Does Electrolysis Produce Electricity.
From www.alamy.com
Electrolysis diagram experiment for education Stock Vector Image & Art Does Electrolysis Produce Electricity An external voltage is applied to drive a nonspontaneous. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction.. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis is the process of using electricity to split water into hydrogen and. An external voltage is applied to drive a nonspontaneous. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction.. Does Electrolysis Produce Electricity.
From pricklynomore.com
The science of electrolysis — Prickly Pear Electrolysis & Skincare Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions. Does Electrolysis Produce Electricity.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Does Electrolysis Produce Electricity Electrolysis is the process of using electricity to split water into hydrogen and. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis, process by which electric current is passed through a substance to effect a chemical change. A chemical change takes place when atoms and ions lose or. For example, electrolysis is a process that involves forcing. Does Electrolysis Produce Electricity.
From circuitlibverla.z21.web.core.windows.net
Circuit Diagram Electrolysis Does Electrolysis Produce Electricity In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. A chemical change takes place when atoms and ions lose or. Electrolysis is the process of using electricity to split water into hydrogen and.. Does Electrolysis Produce Electricity.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in. Does Electrolysis Produce Electricity.
From slidetodoc.com
Electrolysis Electrolysis Process by which an electric current Does Electrolysis Produce Electricity The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. A chemical change takes place when atoms and ions lose or. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. For example, electrolysis is a process that. Does Electrolysis Produce Electricity.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Does Electrolysis Produce Electricity An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis is the process of using electricity. Does Electrolysis Produce Electricity.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Does Electrolysis Produce Electricity Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a. Does Electrolysis Produce Electricity.
From owlcation.com
Electrolysis The Way of the Future Owlcation Does Electrolysis Produce Electricity In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. A chemical change takes place when atoms and ions lose or. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur.. Does Electrolysis Produce Electricity.
From study.com
Electrolysis of Aqueous Solutions Lesson Does Electrolysis Produce Electricity Electrolysis is the process of using electricity to split water into hydrogen and. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis, process by which electric current is passed. Does Electrolysis Produce Electricity.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT Hydrogen Energy PowerPoint Presentation, free download ID6078277 Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using electricity to split water into hydrogen and. In an electrolytic cell, however, the opposite process, called. Does Electrolysis Produce Electricity.
From psu.pb.unizin.org
Electrolysis (17.7) Chemistry 110 Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis is the process of using electricity to split water into hydrogen and. For example, electrolysis is a process that involves forcing electricity through. Does Electrolysis Produce Electricity.
From saylordotorg.github.io
Electrolysis Does Electrolysis Produce Electricity For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis is the process of using electricity to split water into hydrogen. Does Electrolysis Produce Electricity.
From slideplayer.com
Electrolysis ??? Electrolysis is the breakdown of a Does Electrolysis Produce Electricity Electrolysis, process by which electric current is passed through a substance to effect a chemical change. For example, electrolysis is a process that involves forcing electricity through a liquid or solution to cause a reaction to occur. A chemical change takes place when atoms and ions lose or. Electrolysis is the process of using electricity to split water into hydrogen. Does Electrolysis Produce Electricity.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Does Electrolysis Produce Electricity A chemical change takes place when atoms and ions lose or. The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. Electrolysis is the process of using electricity to split water into hydrogen and. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis,. Does Electrolysis Produce Electricity.