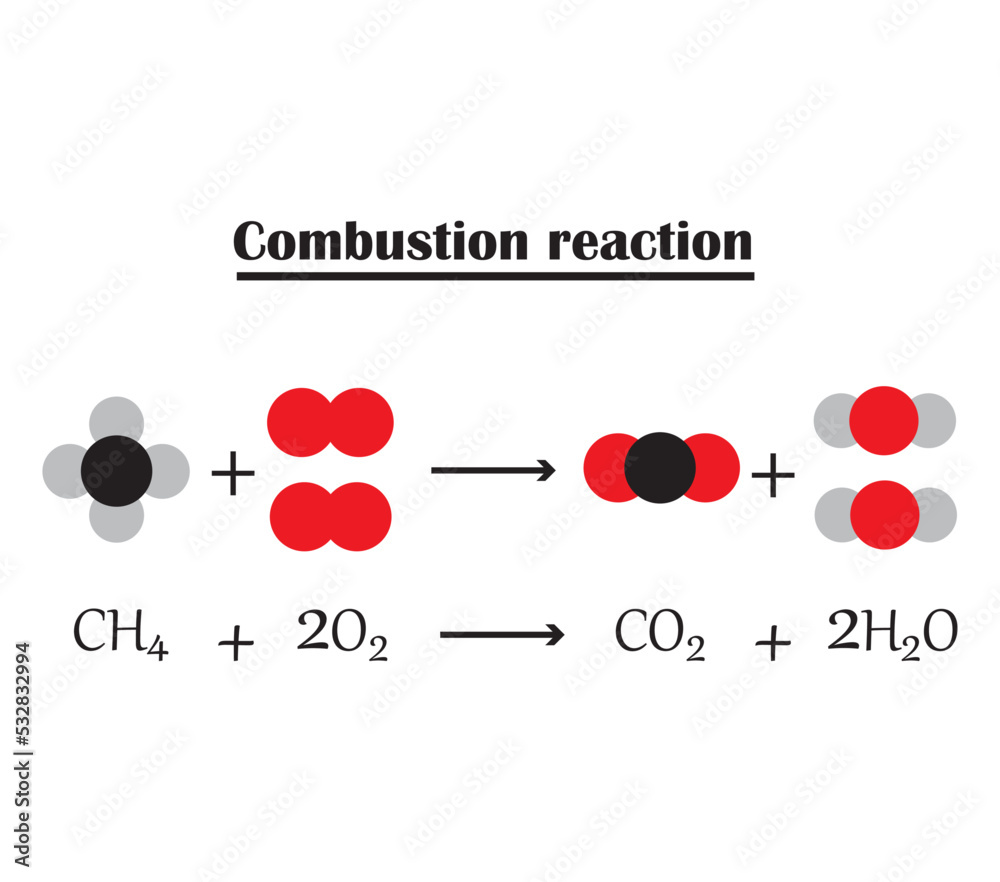
Combustion Reaction General Equation . In this tutorial you will learn what exactly a combustion reaction is and why it is important. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. Combustion reactions must involve \(\ce{o_2}\) as one reactant. here are several examples of balanced equations for combustion reactions. Complete and incomplete combustion reactions. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. A general way to write a combustion reaction is. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. You will also encounter numerous. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. the general equation for the combustion of hydrocarbon is [1]: what is the generic equation for a combustion reaction? C o m b u s t i o n r e a c t i o n.
from stock.adobe.com
A general way to write a combustion reaction is. 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. Combustion reactions must involve \(\ce{o_2}\) as one reactant. Complete and incomplete combustion reactions. the general equation for the combustion of hydrocarbon is [1]: You will also encounter numerous. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction.
Combustion reaction.The chemical formula for the Combustion reaction
Combustion Reaction General Equation Complete and incomplete combustion reactions. here are several examples of balanced equations for combustion reactions. Combustion reactions must involve \(\ce{o_2}\) as one reactant. You will also encounter numerous. 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). C o m b u s t i o n r e a c t i o n. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. the general equation for the combustion of hydrocarbon is [1]: In this tutorial you will learn what exactly a combustion reaction is and why it is important. what is the generic equation for a combustion reaction? a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. A general way to write a combustion reaction is. Complete and incomplete combustion reactions. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant.
From www.youtube.com
Combustion Reactions YouTube Combustion Reaction General Equation what is the generic equation for a combustion reaction? In this tutorial you will learn what exactly a combustion reaction is and why it is important. You will also encounter numerous. Complete and incomplete combustion reactions. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a. Combustion Reaction General Equation.
From www.youtube.com
Complete Combustion Reactions YouTube Combustion Reaction General Equation what is the generic equation for a combustion reaction? there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. You will also encounter numerous. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2. Combustion Reaction General Equation.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free Combustion Reaction General Equation In this tutorial you will learn what exactly a combustion reaction is and why it is important. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. the general equation for the combustion of hydrocarbon is [1]: 2h 2 (g) + o 2 (g) → 2h 2 o. Combustion Reaction General Equation.
From www.slideserve.com
PPT Molecular Formula Calculations Combustion vs. Weight Percent Combustion Reaction General Equation Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. C o m b u s t i o n r e a c t i o n. C 10 h. Combustion Reaction General Equation.
From www.showme.com
Combustion Reaction Science, Chemistry, Chemicalreactions ShowMe Combustion Reaction General Equation the general equation for the combustion of hydrocarbon is [1]: C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. You will also encounter numerous. what is the generic equation for a combustion reaction? Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen. Combustion Reaction General Equation.
From treatybottle13.pythonanywhere.com
Simple Combustion Reaction Octane Physics Formulas Of Class 12 Combustion Reaction General Equation the general equation for the combustion of hydrocarbon is [1]: A general way to write a combustion reaction is. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. here are several examples of balanced equations for combustion reactions. 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). Complete and incomplete combustion reactions. C. Combustion Reaction General Equation.
From wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Combustion Reaction General Equation C o m b u s t i o n r e a c t i o n. A general way to write a combustion reaction is. You will also encounter numerous. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Combustion reactions must involve \(\ce{o_2}\) as one reactant. a combustion. Combustion Reaction General Equation.
From www.youtube.com
AQA A Level Chemistry Organic Chemistry Combustion YouTube Combustion Reaction General Equation In this tutorial you will learn what exactly a combustion reaction is and why it is important. 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). You will also encounter numerous. A general way to write a combustion reaction is. here are several examples of balanced equations for combustion reactions. C 10 h 8 + 12. Combustion Reaction General Equation.
From www.ck12.org
Writing Equations for Combustion Reactions ( Video ) Chemistry CK Combustion Reaction General Equation You will also encounter numerous. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. 2h. Combustion Reaction General Equation.
From www.expii.com
Combustion Reactions — Definition & Examples Expii Combustion Reaction General Equation Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. A general way to write a combustion reaction is. Complete and incomplete combustion reactions. In this tutorial you will learn what exactly a combustion reaction is and why it is important. Complete combustion reactions, also known as clean combustion. Combustion Reaction General Equation.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID Combustion Reaction General Equation there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. . Combustion Reaction General Equation.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube Combustion Reaction General Equation Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Complete and incomplete combustion reactions. what is the generic equation for a combustion reaction? the general equation for the combustion of hydrocarbon is [1]: Complete combustion reactions, also known as. Combustion Reaction General Equation.
From www.youtube.com
CHEMISTRY 101 Combustion reactions, reactions with alkali metals, and Combustion Reaction General Equation 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. A general way to write a combustion reaction is. Complete and incomplete combustion reactions. Note. Combustion Reaction General Equation.
From studylib.net
20 Write a balanced equation for the complete combustion of each Combustion Reaction General Equation C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Combustion reactions must involve \(\ce{o_2}\) as one reactant. You will also encounter numerous. A general way to write a combustion reaction is. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant.. Combustion Reaction General Equation.
From www.youtube.com
CH4 + O2 = CO2 + H2O Balanced Equation (Methane Combustion Reaction Combustion Reaction General Equation 2h 2 (g) + o 2 (g) → 2h 2 o (ℓ). Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. there may. Combustion Reaction General Equation.
From www.slideserve.com
PPT Combustion Basics PowerPoint Presentation, free download ID1194415 Combustion Reaction General Equation Complete and incomplete combustion reactions. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be. Combustion Reaction General Equation.
From www.slideserve.com
PPT 20 Write a balanced equation for the complete combustion of each Combustion Reaction General Equation A general way to write a combustion reaction is. Combustion reactions must involve \(\ce{o_2}\) as one reactant. C o m b u s t i o n r e a c t i o n. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Note that while oxygen gas is always present. Combustion Reaction General Equation.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Combustion Reaction General Equation A general way to write a combustion reaction is. what is the generic equation for a combustion reaction? C o m b u s t i o n r e a c t i o n. the general equation for the combustion of hydrocarbon is [1]: here are several examples of balanced equations for combustion reactions. Combustion. Combustion Reaction General Equation.
From passmyexams.co.uk
Combustion of Alcohols Easy exam revision notes for GSCE Chemistry Combustion Reaction General Equation Complete and incomplete combustion reactions. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. In this tutorial you will learn what exactly a combustion. Combustion Reaction General Equation.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Combustion Reaction General Equation C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. what is the generic equation for a combustion reaction? C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Note that while oxygen gas is always present as a reactant, in the trickier examples, the. Combustion Reaction General Equation.
From www.youtube.com
Chemistry Lesson 37 Combustion Reactions YouTube Combustion Reaction General Equation C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. here are several examples of balanced equations for combustion reactions. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. there may be a coefficient other than one for the. Combustion Reaction General Equation.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Combustion Reaction General Equation Combustion reactions must involve \(\ce{o_2}\) as one reactant. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. C o m b u s t i o n r e a c. Combustion Reaction General Equation.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 Combustion Reaction General Equation You will also encounter numerous. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Complete and incomplete combustion reactions. a. Combustion Reaction General Equation.
From www.thoughtco.com
Main Kinds of Chemical Reactions Combustion Reaction General Equation C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. C o m b u s t i o n r e a c t i o n. Complete and incomplete combustion reactions. In this tutorial you will learn what exactly a combustion reaction is and why it is important. there may. Combustion Reaction General Equation.
From study.com
How to Identify a Combustion Reaction Chemistry Combustion Reaction General Equation Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as. Combustion Reaction General Equation.
From www.chemicals.co.uk
What is Combustion in Chemistry? The Chemistry Blog Combustion Reaction General Equation a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. the general equation for the combustion of hydrocarbon is [1]: 2h 2 (g) +. Combustion Reaction General Equation.
From www.youtube.com
Types of Chemical Equations with Examples Combustion Reaction General Equation Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. Complete and incomplete combustion reactions. C x h y + (x+y/4) o 2 → x co 2 + (y/2) h 2 o. Combustion reactions must involve \(\ce{o_2}\) as one reactant. the general equation for the combustion of hydrocarbon is [1]: Fuel (hydrocarbon) +. Combustion Reaction General Equation.
From sciencenotes.org
Combustion Reaction Definition and Examples Combustion Reaction General Equation a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. what is the generic equation for a combustion reaction? C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. Note that while oxygen gas is always present as. Combustion Reaction General Equation.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Combustion Reaction General Equation Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. the general equation for the combustion of hydrocarbon is [1]: a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. In this tutorial you will learn what exactly a combustion reaction is and why. Combustion Reaction General Equation.
From www.slideserve.com
PPT The combustion process is a chemical reaction whereby fuel is Combustion Reaction General Equation Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (g) burning of naphthalene. C o m b u s t i o n r e a c t i o n. here are several examples of balanced equations for combustion reactions. Note that while oxygen gas is always present as a. Combustion Reaction General Equation.
From www.youtube.com
Balancing Combustion Reactions Chemistry Tutorial YouTube Combustion Reaction General Equation Complete and incomplete combustion reactions. there may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. Complete combustion reactions, also known as clean combustion reactions, is the complete oxidation of the fuel. In this tutorial you will learn what exactly. Combustion Reaction General Equation.
From stock.adobe.com
Combustion reaction.The chemical formula for the Combustion reaction Combustion Reaction General Equation Note that while oxygen gas is always present as a reactant, in the trickier examples, the oxygen comes from another reactant. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h. Combustion Reaction General Equation.
From www.uochemists.com
Combustion Reactions Equations Explanations and Uses UO Chemists Combustion Reaction General Equation C o m b u s t i o n r e a c t i o n. Combustion reactions must involve \(\ce{o_2}\) as one reactant. A general way to write a combustion reaction is. In this tutorial you will learn what exactly a combustion reaction is and why it is important. here are several examples of balanced equations. Combustion Reaction General Equation.
From www.youtube.com
Introduction to Combustion Analysis, Empirical Formula & Molecular Combustion Reaction General Equation Fuel (hydrocarbon) + oxygen = carbon dioxide + water + energy. In this tutorial you will learn what exactly a combustion reaction is and why it is important. Complete and incomplete combustion reactions. You will also encounter numerous. the general equation for the combustion of hydrocarbon is [1]: C 10 h 8 + 12 o 2 → 10 co. Combustion Reaction General Equation.
From www.chemicals.co.uk
What is Combustion in Chemistry? The Chemistry Blog Combustion Reaction General Equation Complete and incomplete combustion reactions. a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. the general equation for the combustion of hydrocarbon is [1]: You will also encounter numerous. C 10 h 8 + 12 o 2 → 10 co 2 + 4 h. Combustion Reaction General Equation.