What Does Indication Mean In Clinical Trials . Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. In clinical trials, indication is used to describe the target population of the study. The indication is often specific to a therapeutic area, which is a. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is to determine whether something.
from anatomisebiostats.com
Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. The indication is often specific to a therapeutic area, which is a. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. In clinical trials, indication is used to describe the target population of the study. The goal is to determine whether something. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies.
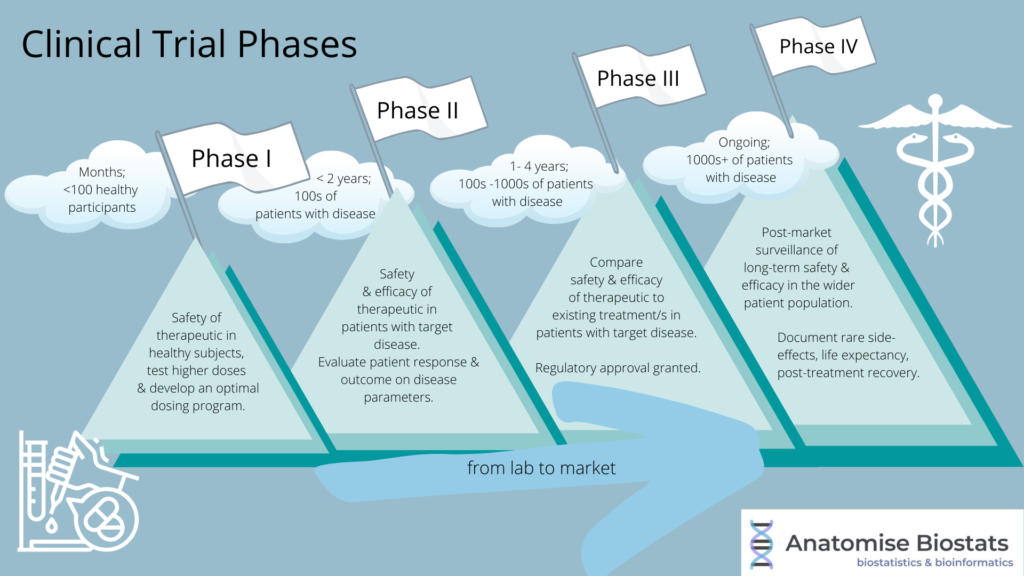
Clinical Trial Phases in Drug Development Biostatistics
What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. The goal is to determine whether something. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. In clinical trials, indication is used to describe the target population of the study. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The indication is often specific to a therapeutic area, which is a. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials.
From crinetics.com
What Are Clinical Trials And Why Are They Important? What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Megan othus and us. What Does Indication Mean In Clinical Trials.
From preferredresearchpartners.com
5 Fast Facts about Clinical Trials Preferred Research Partners What Does Indication Mean In Clinical Trials Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. In clinical trials, indication is used to describe the target population of the study. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and. What Does Indication Mean In Clinical Trials.
From marketbusinessnews.com
What are clinical trials? Definition and examples Market Business News What Does Indication Mean In Clinical Trials In clinical trials, indication is used to describe the target population of the study. The goal is to determine whether something. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The. What Does Indication Mean In Clinical Trials.
From www.hemophilia.org
Understanding Clinical Trials NBDF What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. In clinical trials, indication is used to describe the target population of the study. Clinical trials are a way to test new methods of diagnosing, treating,. What Does Indication Mean In Clinical Trials.
From www.slideteam.net
Clinical Trial Phases With Review And Approval Process Clinical What Does Indication Mean In Clinical Trials Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. In clinical trials, indication is used to describe the target population of the study. Clinical trials are a way to. What Does Indication Mean In Clinical Trials.
From www.slhn.org
Cancer Clinical Trials Cancer Care St. Luke's Cancer Center What Does Indication Mean In Clinical Trials The goal is to determine whether something. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Megan othus and us discuss. What Does Indication Mean In Clinical Trials.
From www.hemophilia.org
Understanding Clinical Trials NBDF What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical. What Does Indication Mean In Clinical Trials.
From www.slideteam.net
Clinical Trial Phases With Endpoints And Timings Clinical Research What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. The treatment discovery process is an iterative. What Does Indication Mean In Clinical Trials.
From www.cbinsights.com
The Evolution Of Clinical Trials The Promise Of AI And The Role Of Big What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Clinical trials are a way to test new. What Does Indication Mean In Clinical Trials.
From cancer.umn.edu
All About Cancer Clinical Trials Trial Safety Measures Masonic What Does Indication Mean In Clinical Trials Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The indication is often specific to a therapeutic area, which is a. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating. What Does Indication Mean In Clinical Trials.
From www.abbvieclinicaltrials.com
What Are The Phases Of Clinical Trials Clinical Trial Phases What Does Indication Mean In Clinical Trials Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. In clinical trials, indication is used to describe the target population of the study. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. The treatment discovery process is. What Does Indication Mean In Clinical Trials.
From www.slideteam.net
Multiple Phases Of Clinical Trial With Clinical Research Trial What Does Indication Mean In Clinical Trials The goal is to determine whether something. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study. The. What Does Indication Mean In Clinical Trials.
From vigyanix.com
How Do Clinical Trials Work From Trial To Treatment [Infographic What Does Indication Mean In Clinical Trials Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. Indication a health problem or disease that is identified as likely to be benefited by a therapy being. What Does Indication Mean In Clinical Trials.
From www.slideteam.net
Multiple Phases Of Clinical Trial With Research Design For What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The treatment discovery process is an iterative process of studying. What Does Indication Mean In Clinical Trials.
From marketbusinessnews.com
What are clinical trials? Definition and examples Market Business News What Does Indication Mean In Clinical Trials The goal is to determine whether something. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. The indication is often specific to a therapeutic area, which is a. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies.. What Does Indication Mean In Clinical Trials.
From anatomisebiostats.com
Clinical Trial Phases in Drug Development Biostatistics What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. In clinical trials, indication is used to describe the target population of the study. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Indication. What Does Indication Mean In Clinical Trials.
From storymd.com
What Are the Phases of Clinical Trials? StoryMD What Does Indication Mean In Clinical Trials In clinical trials, indication is used to describe the target population of the study. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The indication is often specific to a therapeutic area,. What Does Indication Mean In Clinical Trials.
From www.lymphoma.org.au
Understanding Clinical Trials Lymphoma Australia What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. The goal is to determine whether something. Clinical. What Does Indication Mean In Clinical Trials.
From www.anzgog.org.au
How does a clinical trial work ANZGOG What Does Indication Mean In Clinical Trials Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Ich e8 provides an overall introduction to. What Does Indication Mean In Clinical Trials.
From www.ripplescience.com
The NIH Clinical Trial Definition Change What do Clinical Best What Does Indication Mean In Clinical Trials In clinical trials, indication is used to describe the target population of the study. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Megan othus and us. What Does Indication Mean In Clinical Trials.
From www.openmed.co.in
Phases of Clinical Trial SUMMARY What Does Indication Mean In Clinical Trials Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. The goal is to determine whether something. The indication is often specific to a therapeutic area, which is a. Indication a health problem or disease that is identified as. What Does Indication Mean In Clinical Trials.
From www.researchgate.net
Clinical trial progression and therapeutic indication for the new What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study.. What Does Indication Mean In Clinical Trials.
From blog.zoomrx.com
Balancing Ethics and Innovation in Clinical Trials A ZoomRx Resource What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. The indication is often specific to a therapeutic area, which is a. The goal is to determine whether something. In clinical trials, indication is used to describe the target population of the study. Ich e8 provides an overall introduction to clinical development,. What Does Indication Mean In Clinical Trials.
From acromegalysupport.com
Clinical Trials Part 3 Phases of Clinical Trials and What Happens in Each What Does Indication Mean In Clinical Trials Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Indication a health problem or disease that is identified as likely to be benefited by a therapy being. What Does Indication Mean In Clinical Trials.
From www.alzheimersresearchuk.org
Clinical trials Alzheimer's Research UK What Does Indication Mean In Clinical Trials Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. In clinical trials, indication is used to describe the target population of the study. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is to. What Does Indication Mean In Clinical Trials.
From lustgarten.org
Clinical Trial Phases What Does Indication Mean In Clinical Trials Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. The goal is to determine whether something. The treatment discovery process is. What Does Indication Mean In Clinical Trials.
From www.universityofcalifornia.edu
A new phase for clinical trials University of California What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. The goal is to determine whether something. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical. What Does Indication Mean In Clinical Trials.
From www.researchgate.net
Indications addressed by gene therapy clinical trials Download What Does Indication Mean In Clinical Trials The goal is to determine whether something. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. The indication is often specific to a therapeutic area, which is a. Indication a health problem or disease that is identified as likely to be benefited by a therapy being studied in clinical trials. Megan. What Does Indication Mean In Clinical Trials.
From www.cancertherapyadvisor.com
Risks and Benefits of Phase 1 Clinical Trial Participation Cancer What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. The indication is often specific to a therapeutic area, which is a. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study.. What Does Indication Mean In Clinical Trials.
From www.slideteam.net
Multiple Phases Of Clinical Trial With Milestones Research Design For What Does Indication Mean In Clinical Trials Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study. The goal is to determine whether something. The treatment discovery process is an iterative process. What Does Indication Mean In Clinical Trials.
From owise.uk
Clinical Trials for Breast Cancer Our A to Z Guide OWise UK What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. Indication a health problem or disease that is identified as likely to be benefited. What Does Indication Mean In Clinical Trials.
From www.aotearoatrials.nz
Participants Aotearoa Clinical Trials, Auckland New Zealand What Does Indication Mean In Clinical Trials The indication is often specific to a therapeutic area, which is a. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. In clinical trials, indication is used to describe the target population of the study. The goal is to determine whether something. The treatment discovery process is an iterative process of studying a. What Does Indication Mean In Clinical Trials.
From www.researchgate.net
Immunotherapy trials with indication of their phase, clinicalTrials.gov What Does Indication Mean In Clinical Trials Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is. What Does Indication Mean In Clinical Trials.
From diagramresearch.com
Phases In Clinical Trials Explained by CRO Diagram Research What Does Indication Mean In Clinical Trials Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The indication is often specific to a therapeutic area, which is a. The goal is to determine whether something. Megan othus and us discuss complexities in clinical trials interpretation including the challenge of false. Indication a health problem or disease that is identified as. What Does Indication Mean In Clinical Trials.
From mavink.com
Phases Of Clinical Trials Chart What Does Indication Mean In Clinical Trials The treatment discovery process is an iterative process of studying a disease, hypothesizing and developing treatments, evaluating those. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. In clinical trials, indication is used to describe the target population of the study. The goal. What Does Indication Mean In Clinical Trials.