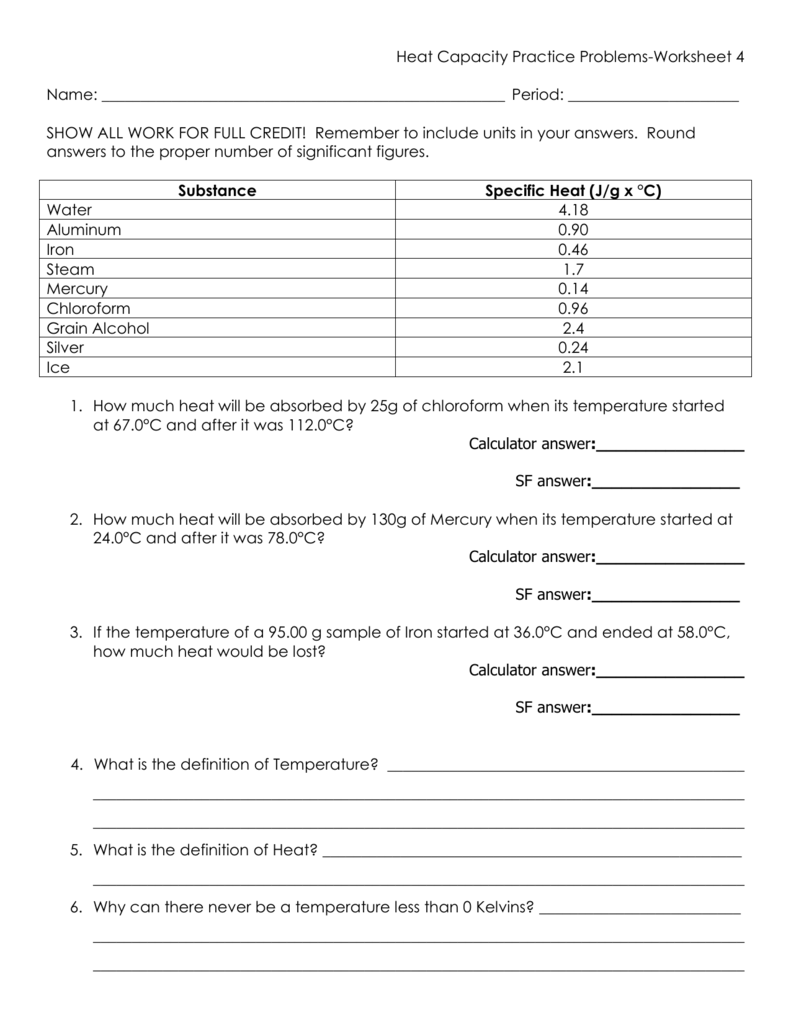
Heat Capacity Practice Questions . Includes full solutions and score reporting. Practice problems on heat capacity and specific heat. Latent heat and specific heat capacity questions. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? The specific heat of water is 4.18 j/g°c (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Calculate the change in enthalpy for the reaction in kj. How much water at 32°c. I record the energy transferred into the metal as 4.5 × 1 0 3. Examples of how to determine, the heat, heat capacity, and change of temperature. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c.
from studylib.net
Latent heat and specific heat capacity questions. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? Practice problems on heat capacity and specific heat. Calculate the change in enthalpy for the reaction in kj. Examples of how to determine, the heat, heat capacity, and change of temperature. I record the energy transferred into the metal as 4.5 × 1 0 3. The specific heat of water is 4.18 j/g°c How much water at 32°c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Includes full solutions and score reporting.
Heat Capacity Practice Problems
Heat Capacity Practice Questions How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. Calculate the change in enthalpy for the reaction in kj. Practice problems on heat capacity and specific heat. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? How much water at 32°c. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Examples of how to determine, the heat, heat capacity, and change of temperature. I record the energy transferred into the metal as 4.5 × 1 0 3. Includes full solutions and score reporting. The specific heat of water is 4.18 j/g°c (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.).
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Heat Capacity Practice Questions Includes full solutions and score reporting. Practice problems on heat capacity and specific heat. Examples of how to determine, the heat, heat capacity, and change of temperature. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. The specific heat of water is 4.18 j/g°c How much water at 50°c is needed to. Heat Capacity Practice Questions.
From www.studocu.com
Heat Capacity Calorimetry Worksheet answers NAME_________________PER________ Heat Studocu Heat Capacity Practice Questions Practice problems on heat capacity and specific heat. Calculate the change in enthalpy for the reaction in kj. Examples of how to determine, the heat, heat capacity, and change of temperature. The specific heat of water is 4.18 j/g°c I record the energy transferred into the metal as 4.5 × 1 0 3. How many grams of water would require. Heat Capacity Practice Questions.
From www.alchemical.org
PWHS Thermodynamics Specific Heat Worksheet Heat Capacity Practice Questions Includes full solutions and score reporting. I record the energy transferred into the metal as 4.5 × 1 0 3. How much water at 32°c. Examples of how to determine, the heat, heat capacity, and change of temperature. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? The specific heat of water is. Heat Capacity Practice Questions.
From studylib.net
Specific Heat Capacity Practice Problems Heat Capacity Practice Questions Examples of how to determine, the heat, heat capacity, and change of temperature. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. I record the energy transferred into the metal as 4.5 × 1 0 3. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c?. Heat Capacity Practice Questions.
From www.studypool.com
SOLUTION Thermal energy heat and specific heat capacity pratice word problem questions with Heat Capacity Practice Questions Calculate the change in enthalpy for the reaction in kj. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? How much water at 50°c is needed to just melt 2.2 kg of ice. Heat Capacity Practice Questions.
From gcsephysicsninja.com
35. Specific heat capacity equation Heat Capacity Practice Questions Latent heat and specific heat capacity questions. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. The specific heat of water is 4.18 j/g°c Includes full solutions and score reporting. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? I record the. Heat Capacity Practice Questions.
From www.worksheetsgo.com
Specific Heat Worksheets WorksheetsGO Heat Capacity Practice Questions I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Includes full solutions and score reporting. Latent heat and specific heat capacity questions. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? Examples of how to determine, the heat, heat capacity, and change. Heat Capacity Practice Questions.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our Comprehensive Questions Heat Capacity Practice Questions Practice problems on heat capacity and specific heat. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? I record the energy transferred into the metal as 4.5 × 1 0 3. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. (use 1.0. Heat Capacity Practice Questions.
From printablefullwinford.z19.web.core.windows.net
Calculating Heat And Specific Heat Worksheets Heat Capacity Practice Questions Includes full solutions and score reporting. Examples of how to determine, the heat, heat capacity, and change of temperature. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? The specific heat of water is 4.18 j/g°c Practice problems on heat capacity and specific heat. I heat 0.20 kg of an unknown metal, so. Heat Capacity Practice Questions.
From studylib.net
Heat Capacity and Specific Heat Worksheet 1 3/3/04 12641 PM Heat Capacity Practice Questions How much water at 32°c. I record the energy transferred into the metal as 4.5 × 1 0 3. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? Practice problems on heat capacity and specific heat. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the. Heat Capacity Practice Questions.
From www.youtube.com
Specific Latent Heat and Heat Capacity Exam Question YouTube Heat Capacity Practice Questions Includes full solutions and score reporting. Latent heat and specific heat capacity questions. I record the energy transferred into the metal as 4.5 × 1 0 3. Examples of how to determine, the heat, heat capacity, and change of temperature. Practice problems on heat capacity and specific heat. I heat 0.20 kg of an unknown metal, so that the temperature. Heat Capacity Practice Questions.
From www.docsity.com
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet Exercises Physics Heat Capacity Practice Questions Practice problems on heat capacity and specific heat. Examples of how to determine, the heat, heat capacity, and change of temperature. Latent heat and specific heat capacity questions. Calculate the change in enthalpy for the reaction in kj. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. I record the energy transferred. Heat Capacity Practice Questions.
From www.scribd.com
Level Past Paper Questions Hysics O P TOPIC8 Heat Capacity PAPER1 Multiple Choice PDF Heat Capacity Practice Questions Includes full solutions and score reporting. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). How much water at 32°c. Practice problems on heat capacity and specific heat. How many grams of water would require. Heat Capacity Practice Questions.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and latent heats Heat Capacity Practice Questions Includes full solutions and score reporting. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. The specific heat of water is 4.18 j/g°c Latent heat and specific heat capacity questions. Calculate the change. Heat Capacity Practice Questions.
From www.worksheeto.com
10 Science Worksheets On Heat / Heat Capacity Practice Questions How much water at 32°c. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Examples of how to determine, the heat, heat capacity, and change of temperature. I record the energy transferred into the metal as 4.5 × 1 0 3. I heat 0.20 kg of an unknown metal, so that the. Heat Capacity Practice Questions.
From www.studocu.com
Specific Heat Practice Problems Specific Heat Worksheet C = T/m ̈T, ZheUe T = heaW eneUg, m Heat Capacity Practice Questions I record the energy transferred into the metal as 4.5 × 1 0 3. Latent heat and specific heat capacity questions. How much water at 32°c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to. Heat Capacity Practice Questions.
From www.youtube.com
Chemistry Practice Problems Heat and Specific Heat YouTube Heat Capacity Practice Questions (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Includes full solutions and score reporting. The specific heat of. Heat Capacity Practice Questions.
From worksheettantarow9.z21.web.core.windows.net
Heat Practice Problems Worksheets Heat Capacity Practice Questions I record the energy transferred into the metal as 4.5 × 1 0 3. How much water at 32°c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). I heat 0.20 kg of an unknown metal,. Heat Capacity Practice Questions.
From studylib.net
Specific Heat Capacity and Latent Heat Questions Heat Capacity Practice Questions Practice problems on heat capacity and specific heat. Includes full solutions and score reporting. How much water at 32°c. Examples of how to determine, the heat, heat capacity, and change of temperature. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Latent heat and specific heat capacity questions. Calculate the change in. Heat Capacity Practice Questions.
From www.studypool.com
SOLUTION 6chap heat capacity specific heat worksheet Studypool Heat Capacity Practice Questions I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Examples of how to determine, the heat, heat capacity, and change of temperature. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. Practice problems on heat capacity and specific. Heat Capacity Practice Questions.
From obropolox.blogspot.com
40 specific heat capacity worksheet answers Worksheet Resource Heat Capacity Practice Questions I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Practice problems on heat capacity and specific heat. How much water at 32°c. The specific heat of water is 4.18 j/g°c Includes full solutions and score reporting. Latent heat and specific heat capacity questions. Calculate the change in enthalpy for the reaction in. Heat Capacity Practice Questions.
From studylib.net
Specific Heat Capacity Practice Problems Heat Capacity Practice Questions Calculate the change in enthalpy for the reaction in kj. Practice problems on heat capacity and specific heat. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Includes full solutions and score reporting. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). I heat 0.20. Heat Capacity Practice Questions.
From www.tes.com
Specific Heat Capacity Required Practical Exam Style Questions Teaching Resources Heat Capacity Practice Questions How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? Examples of how to determine, the heat, heat capacity, and change of temperature. How much water at 32°c. I record the energy transferred into the metal as 4.5 × 1 0 3. (use 1.0 g/ml as the density of the solution. Heat Capacity Practice Questions.
From studylib.net
Heat Capacity Practice Problems Heat Capacity Practice Questions How much water at 32°c. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Latent heat and specific heat. Heat Capacity Practice Questions.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our Comprehensive Questions Heat Capacity Practice Questions I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Includes full solutions and score reporting. The specific heat of water is 4.18 j/g°c (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Practice problems on heat capacity and specific heat. I record the energy. Heat Capacity Practice Questions.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and latent heats Heat Capacity Practice Questions (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Includes full solutions and score reporting. The specific heat of water is 4.18 j/g°c How many grams of water would require 2200 joules of heat to. Heat Capacity Practice Questions.
From www.tes.com
Specific Heat Capacity Practice Calculations Teaching Resources Heat Capacity Practice Questions How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? How much water at 32°c. Calculate the change in enthalpy for the reaction in kj. (use 1.0 g/ml. Heat Capacity Practice Questions.
From www.tes.com
GCSE Physics Worksheet Specific Heat Capacity, definition, formula, Q&A Teaching Resources Heat Capacity Practice Questions How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. How much water at 32°c. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? The specific heat of water is 4.18 j/g°c (use 1.0 g/ml as the. Heat Capacity Practice Questions.
From www.tes.com
GCSE/IGCSE Physics Specific Heat Capacity SelfAssessment Questions & Answers [2023 Heat Capacity Practice Questions The specific heat of water is 4.18 j/g°c How much water at 32°c. Latent heat and specific heat capacity questions. I record the energy transferred into the metal as 4.5 × 1 0 3. (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Examples of how to determine, the heat, heat capacity,. Heat Capacity Practice Questions.
From studylib.net
Heat Capacity Practice Heat Capacity Practice Questions (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Latent heat and specific heat capacity questions. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Includes full solutions. Heat Capacity Practice Questions.
From www.studocu.com
SpecificHeatPracticeProblems Specific Heat Worksheet C = T/m ̈T, ZheUe T = heaW eneUg, m Heat Capacity Practice Questions (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Calculate the change in enthalpy for the reaction in kj. Examples of how to determine, the heat, heat capacity, and change of temperature. The specific heat of water is 4.18 j/g°c How much water at 32°c. Includes full solutions and score reporting. How. Heat Capacity Practice Questions.
From studylib.net
Specific Heat Capacity Questions Heat Capacity Practice Questions (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). Includes full solutions and score reporting. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. The specific heat of. Heat Capacity Practice Questions.
From www.chegg.com
Solved 9 SPECIFIC HEAT CAPACITY BY THE METHOD OF MIXTURES Heat Capacity Practice Questions I record the energy transferred into the metal as 4.5 × 1 0 3. I heat 0.20 kg of an unknown metal, so that the temperature increases by 45 ∘ c. Practice problems on heat capacity and specific heat. Calculate the change in enthalpy for the reaction in kj. How many grams of water would require 2200 joules of heat. Heat Capacity Practice Questions.
From docs.google.com
Specific Heat Capacity Questions 1 (with answers) Google Docs Heat Capacity Practice Questions How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. How many grams of water would require 2200 joules of heat to raise its temperature from 34°c to 100°c? Includes full solutions and score reporting. Calculate the change in enthalpy for the reaction in kj. How much. Heat Capacity Practice Questions.
From obropolox.blogspot.com
40 specific heat capacity worksheet answers Worksheet Resource Heat Capacity Practice Questions Practice problems on heat capacity and specific heat. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? (use 1.0 g/ml as the density of the solution and 4.18 j/g°c as the specific heat capacity.). The specific heat of water is 4.18 j/g°c I heat 0.20 kg of an unknown metal, so that the. Heat Capacity Practice Questions.