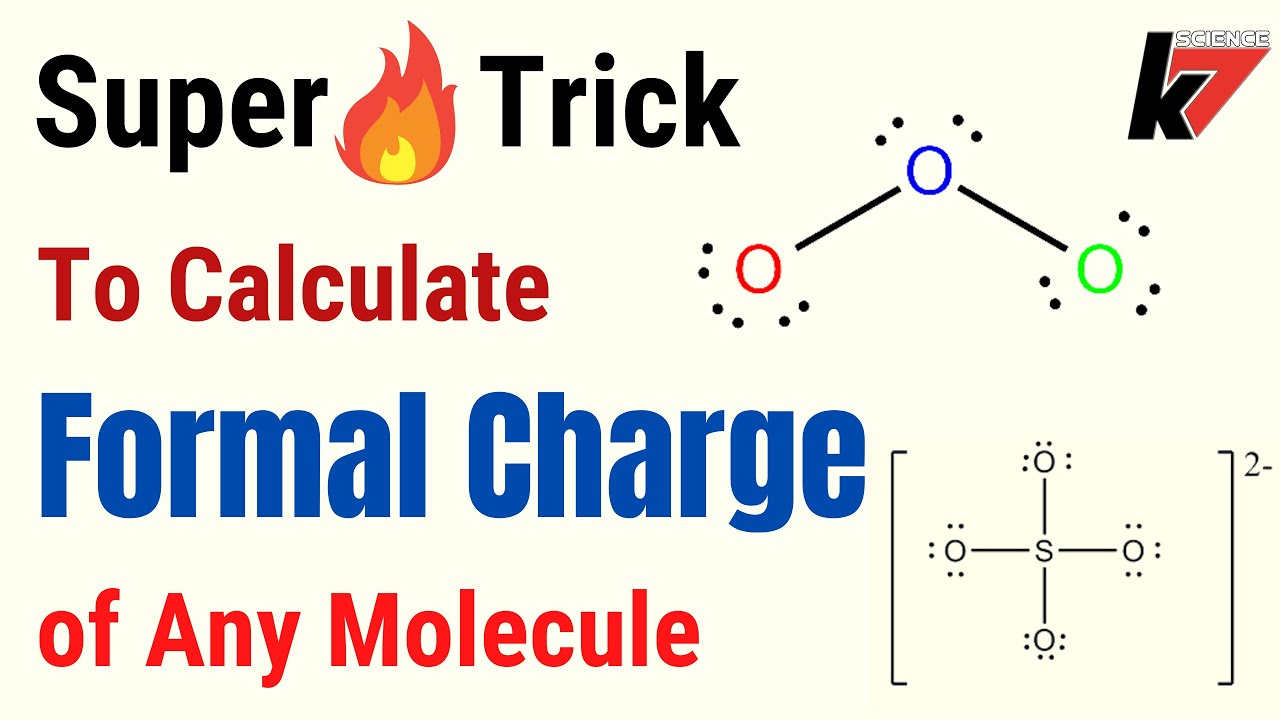
How To Get The Overall Charge Of An Atom . However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. In this article, we will. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. You can work out what this charge will be based on the periodic table group. here's the formula for figuring out the formal charge of an atom: how to determine the charge of an ion, element, and atom examples and. describe the locations, charges, and masses of the three main subatomic particles. how to calculate the charge of an atom using the number of protons and electrons. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom.
from www.youtube.com
most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. how to determine the charge of an ion, element, and atom examples and. how to calculate the charge of an atom using the number of protons and electrons. In this article, we will. You can work out what this charge will be based on the periodic table group. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. here's the formula for figuring out the formal charge of an atom: describe the locations, charges, and masses of the three main subatomic particles.
How to find formal charge formal charge of an atom calculate in
How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: here's the formula for figuring out the formal charge of an atom: how to determine the charge of an ion, element, and atom examples and. describe the locations, charges, and masses of the three main subatomic particles. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. how to calculate the charge of an atom using the number of protons and electrons. You can work out what this charge will be based on the periodic table group. In this article, we will.
From guidefixaturdirosrv.z22.web.core.windows.net
How To Determine An Atoms Charge How To Get The Overall Charge Of An Atom how to determine the charge of an ion, element, and atom examples and. In this article, we will. describe the locations, charges, and masses of the three main subatomic particles. You can work out what this charge will be based on the periodic table group. here's the formula for figuring out the formal charge of an atom:. How To Get The Overall Charge Of An Atom.
From sciencing.com
How to Calculate the Charge of an Ion Sciencing How To Get The Overall Charge Of An Atom In this article, we will. You can work out what this charge will be based on the periodic table group. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. how to determine the charge of an ion, element, and atom examples and. most of the time, the charge. How To Get The Overall Charge Of An Atom.
From www.numerade.com
SOLVED Determine the formal charge on each atom in the structure How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: You can work out what this charge will be based on the periodic table group. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. In this article, we will. describe the locations, charges, and masses of. How To Get The Overall Charge Of An Atom.
From manualdataaerography.z13.web.core.windows.net
How To Determine Charge Of Atom How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. You can work out what this charge will be based on the periodic table group. the basic principle involves determining. How To Get The Overall Charge Of An Atom.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. describe the locations,. How To Get The Overall Charge Of An Atom.
From www.slideserve.com
PPT Chapter 3 Section 1 Notes PowerPoint Presentation, free download How To Get The Overall Charge Of An Atom describe the locations, charges, and masses of the three main subatomic particles. how to calculate the charge of an atom using the number of protons and electrons. In this article, we will. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. However, some atoms can form ions,. How To Get The Overall Charge Of An Atom.
From www.chegg.com
Solved Determine the formal charge on each atom in the How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. how to calculate the. How To Get The Overall Charge Of An Atom.
From schematicskeifanoqxw5.z13.web.core.windows.net
How To Determine Charge Of Atom How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: You can work out what this charge will be based on the periodic table group. how to determine the charge of an ion, element, and atom examples and. how to calculate the charge of an atom using the number of protons and electrons. most. How To Get The Overall Charge Of An Atom.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry How To Get The Overall Charge Of An Atom describe the locations, charges, and masses of the three main subatomic particles. In this article, we will. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. how to. How To Get The Overall Charge Of An Atom.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview How To Get The Overall Charge Of An Atom However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. here's the formula for figuring out the formal charge of an atom: the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. most of the time,. How To Get The Overall Charge Of An Atom.
From quizspattering.z21.web.core.windows.net
How To Determine Charge How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: In this article, we will. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. You can work out what this charge will be based on the periodic table group. most of the. How To Get The Overall Charge Of An Atom.
From www.youtube.com
How to find formal charge formal charge of an atom calculate in How To Get The Overall Charge Of An Atom However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. In this article, we will. how to determine the charge of an ion, element, and atom examples and. describe the locations, charges, and masses of the three main subatomic particles. most of the time, the charge of elements. How To Get The Overall Charge Of An Atom.
From circuitchaphazibh.z14.web.core.windows.net
How To Determine An Atoms Charge How To Get The Overall Charge Of An Atom the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. You can work out what this charge will be based on the periodic table group. In this article, we will. how to calculate the charge of an atom using the number of protons and electrons. . How To Get The Overall Charge Of An Atom.
From www.numerade.com
SOLVED 'Determine the formal charge on each atom in the structure How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. describe the locations, charges, and masses of the three main subatomic particles. how to determine the charge of an ion, element, and atom examples and. In this article, we will. the basic principle involves determining the difference. How To Get The Overall Charge Of An Atom.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint How To Get The Overall Charge Of An Atom here's the formula for figuring out the formal charge of an atom: how to calculate the charge of an atom using the number of protons and electrons. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. In this article, we will. describe the locations, charges, and masses. How To Get The Overall Charge Of An Atom.
From clarkfvspears.blogspot.com
How to Calculate Formal Charge ClarkfvSpears How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. how to determine the charge of an ion, element, and atom examples and. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. You can work. How To Get The Overall Charge Of An Atom.
From www.slideserve.com
PPT IONS AND THEIR COMPOUNDS PowerPoint Presentation ID3961680 How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. You can work out what this charge will be based on the periodic table group. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. how. How To Get The Overall Charge Of An Atom.
From schematicskeifanoqxw5.z13.web.core.windows.net
How To Determine An Atoms Charge How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. describe the locations, charges, and masses of the three main subatomic particles. how to determine the charge of an ion, element, and atom examples and. You can work out what this charge will be based on the periodic. How To Get The Overall Charge Of An Atom.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom How To Get The Overall Charge Of An Atom However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. how to determine the charge of an ion, element, and atom examples and. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. describe the locations,. How To Get The Overall Charge Of An Atom.
From www.youtube.com
Calculating charge using Electrons and Protons YouTube How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. You can work out what this charge will be based on the periodic table group. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. describe. How To Get The Overall Charge Of An Atom.
From www.youtube.com
How To Calculate The Formal Charge of an Atom Chemistry YouTube How To Get The Overall Charge Of An Atom You can work out what this charge will be based on the periodic table group. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. how to calculate the charge of an atom using the number of protons and electrons. how to determine the charge of an ion, element,. How To Get The Overall Charge Of An Atom.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? How To Get The Overall Charge Of An Atom In this article, we will. You can work out what this charge will be based on the periodic table group. how to determine the charge of an ion, element, and atom examples and. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. how to calculate the charge. How To Get The Overall Charge Of An Atom.
From www.youtube.com
Atoms, nuclei and specific charge YouTube How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. describe the locations, charges, and masses of the three main subatomic particles. You can work out what this charge will be based on the periodic table group. In this article, we will. However, some atoms can form ions, gaining. How To Get The Overall Charge Of An Atom.
From www.chegg.com
Solved 3 atom and (ii) the overall charge on each species. How To Get The Overall Charge Of An Atom how to determine the charge of an ion, element, and atom examples and. how to calculate the charge of an atom using the number of protons and electrons. here's the formula for figuring out the formal charge of an atom: You can work out what this charge will be based on the periodic table group. In this. How To Get The Overall Charge Of An Atom.
From www.slideserve.com
PPT Atoms PowerPoint Presentation, free download ID2439568 How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. describe the locations, charges, and masses of the three main subatomic particles. In this article, we will. how to. How To Get The Overall Charge Of An Atom.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce How To Get The Overall Charge Of An Atom how to calculate the charge of an atom using the number of protons and electrons. describe the locations, charges, and masses of the three main subatomic particles. here's the formula for figuring out the formal charge of an atom: how to determine the charge of an ion, element, and atom examples and. You can work out. How To Get The Overall Charge Of An Atom.
From www.chegg.com
Solved Determine the formal charge on each atom in the How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. You can work out what this charge will be based on the periodic table group. describe the locations, charges, and masses of the three main subatomic particles. here's the formula for figuring out the formal charge of an. How To Get The Overall Charge Of An Atom.
From www.slideserve.com
PPT Chapter 3 Section 1 Notes PowerPoint Presentation, free download How To Get The Overall Charge Of An Atom the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. describe the locations, charges, and masses of the three main subatomic particles. how to determine the charge of an ion, element, and atom examples and. However, some atoms can form ions, gaining or losing electrons. How To Get The Overall Charge Of An Atom.
From frecetovbpschematic.z4.web.core.windows.net
How To Determine An Atoms Charge How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. You can work out what this charge will be based on the periodic table group. However, some. How To Get The Overall Charge Of An Atom.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry How To Get The Overall Charge Of An Atom most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. In this article, we will. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. how to calculate the charge of an atom using the number of protons and electrons.. How To Get The Overall Charge Of An Atom.
From www.breakingatom.com
Nuclear Charge How To Get The Overall Charge Of An Atom how to determine the charge of an ion, element, and atom examples and. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. You can work out what this charge will be based on the periodic table group. most of the time, the charge of. How To Get The Overall Charge Of An Atom.
From www.tvrn.co.uk
What is the overall electrical charge of an atom with an equal number How To Get The Overall Charge Of An Atom how to determine the charge of an ion, element, and atom examples and. However, some atoms can form ions, gaining or losing electrons to pick up a net negative or positive charge. here's the formula for figuring out the formal charge of an atom: how to calculate the charge of an atom using the number of protons. How To Get The Overall Charge Of An Atom.
From slideplayer.com
Atoms and the Periodic Table ppt download How To Get The Overall Charge Of An Atom describe the locations, charges, and masses of the three main subatomic particles. how to calculate the charge of an atom using the number of protons and electrons. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. the basic principle involves determining the difference in the number. How To Get The Overall Charge Of An Atom.
From www.thetechedvocate.org
How to calculate formal charge on a lewis structure The Tech Edvocate How To Get The Overall Charge Of An Atom You can work out what this charge will be based on the periodic table group. how to calculate the charge of an atom using the number of protons and electrons. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. how to determine the charge of an ion,. How To Get The Overall Charge Of An Atom.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent How To Get The Overall Charge Of An Atom how to determine the charge of an ion, element, and atom examples and. the basic principle involves determining the difference in the number of protons and electrons to calculate the overall charge of an atom. most of the time, the charge of elements is zero, because they have equal numbers of protons and electrons. You can work. How To Get The Overall Charge Of An Atom.