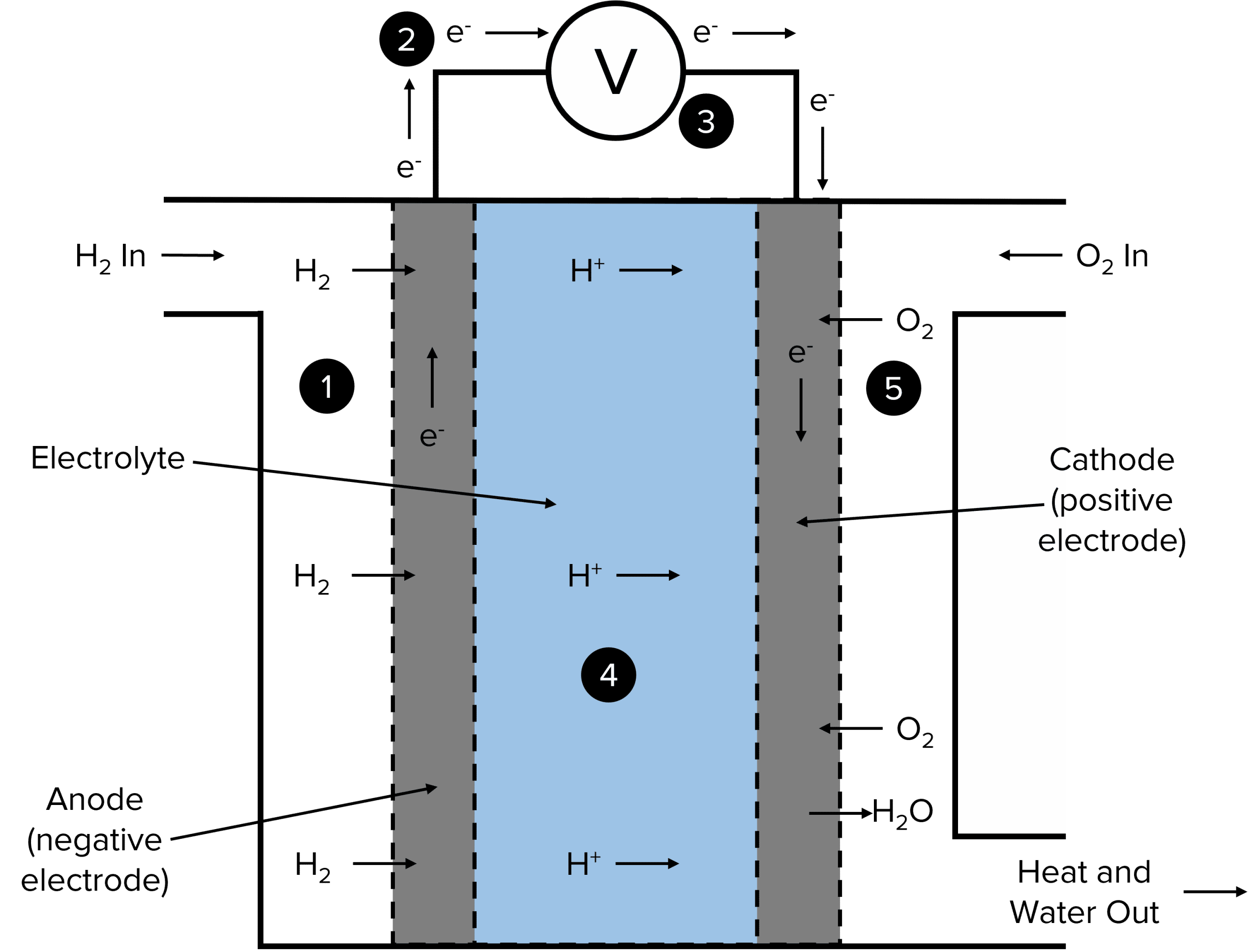
Fuel Cells Chemistry Notes . a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. In the presence of two.
from mmerevise.co.uk
A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. In the presence of two. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other.
Electrochemical Cells Worksheets and Revision MME
Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and. Fuel Cells Chemistry Notes.
From www.youtube.com
Fuel Cell O level / IGCSE Chemistry YouTube Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at. Fuel Cells Chemistry Notes.
From www.tes.com
Chemical Cells and Fuel Cells Mind map for AQA GCSE Chemistry for 2018 Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell is a tiny device, capable of generating electricity. Fuel Cells Chemistry Notes.
From www.pinterest.co.uk
FREE GCSE Chemistry (Science) Hydrogen Fuel Cells Practice Exam Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. In the presence of two. fuel cells produce a voltage close voltage the potential difference. Fuel Cells Chemistry Notes.
From cmsw.mit.edu
» Small Steps and Giant Leaps in Fuel Cell Technologies Angles / 2022 Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. In the presence. Fuel Cells Chemistry Notes.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Fuel Cells Chemistry Notes In the presence of two. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell is a tiny device, capable of generating electricity. Fuel Cells Chemistry Notes.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is an electrochemical cell in which a fuel donates electrons at. Fuel Cells Chemistry Notes.
From www.scribd.com
Fuel Cell Chemistry Presentation PDF Fuel Cell Hydrogen Fuel Cells Chemistry Notes In the presence of two. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is an electrochemical cell in which a fuel donates electrons at. Fuel Cells Chemistry Notes.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the. Fuel Cells Chemistry Notes.
From www.thinkswap.com
Fuel Cells Chemistry Year 12 VCE Thinkswap Fuel Cells Chemistry Notes a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition. Fuel Cells Chemistry Notes.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. fuel cells are similar to batteries in that they generate an electrical current, but require continuous. Fuel Cells Chemistry Notes.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. fuel cells produce a voltage close voltage the potential difference across. Fuel Cells Chemistry Notes.
From www.scribd.com
Mainanieee PDF Fuel Cell Chemistry Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell is a galvanic cell that requires a constant external supply of. Fuel Cells Chemistry Notes.
From www.mdpi.com
Applied Sciences Free FullText Portable Prototype of Hydrogen Fuel Fuel Cells Chemistry Notes a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries in that they generate an. Fuel Cells Chemistry Notes.
From www.biologic.net
Electrochemical characterisation of fuel cells and electrolysers Fuel Cells Chemistry Notes a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. fuel cells are similar to batteries in that they generate an electrical current, but require. Fuel Cells Chemistry Notes.
From www.yumpu.com
Fuel Cell Formulary_A4.pdf ZSW Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell like this will continue to operate and produce electrical energy as long as a. Fuel Cells Chemistry Notes.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell like this will continue to operate and produce electrical energy as long as a. Fuel Cells Chemistry Notes.
From www.studocu.com
Fuels Notes CHEMISTRY CHAPTER 2 FUELS Aspect of the Study Design Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell is an electrochemical cell in which a fuel donates electrons at one. Fuel Cells Chemistry Notes.
From chem.libretexts.org
17.5 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells Chemistry Notes a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is a tiny device, capable of generating electricity by force of a. Fuel Cells Chemistry Notes.
From www.youtube.com
fuel cells electrochemistry 2 class 12 chemistry subject notes lectures Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of. Fuel Cells Chemistry Notes.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. In the presence of two. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and. Fuel Cells Chemistry Notes.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is an electrochemical cell in which a fuel donates electrons. Fuel Cells Chemistry Notes.
From www.savemyexams.com
Fuel Cells HL IB Chemistry Revision Notes 2025 Save My Exams Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. . Fuel Cells Chemistry Notes.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the. Fuel Cells Chemistry Notes.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Fuel Cells Chemistry Notes In the presence of two. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is a tiny device, capable. Fuel Cells Chemistry Notes.
From www.pinterest.com.au
Electrochemistry, featuring electrolysis and fuel cells. Chemistry Fuel Cells Chemistry Notes In the presence of two. fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell is a galvanic cell that requires a constant external supply of reactants. Fuel Cells Chemistry Notes.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell is an electrochemical cell in which a fuel donates electrons at one. Fuel Cells Chemistry Notes.
From www.thinkswap.com
Electrochemical and Fuel Cells Chemistry Year 12 WACE Thinkswap Fuel Cells Chemistry Notes fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries in that they generate an electrical current, but require continuous. Fuel Cells Chemistry Notes.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cells Chemistry Notes a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell like this will continue to operate and produce electrical energy as long as a. Fuel Cells Chemistry Notes.
From www.scribd.com
Module3 and Module4 Notes PDF Fuel Cell Chemistry Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. In the presence of two. fuel cells produce a voltage close voltage the potential difference across a cell, electrical supply or electrical component. a fuel cell is an electrochemical cell in which a fuel. Fuel Cells Chemistry Notes.
From mavink.com
Solid Oxide Fuel Cell Applications Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. In the presence of two. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. A fuel cell is a galvanic cell. Fuel Cells Chemistry Notes.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Fuel Cells Chemistry Notes a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. In the presence of two. a fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. fuel cells are similar to batteries in that they generate an electrical. Fuel Cells Chemistry Notes.
From www.youtube.com
3.17Fuel cell class 12th,Electrochemistry YouTube Fuel Cells Chemistry Notes In the presence of two. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells are similar to batteries. Fuel Cells Chemistry Notes.
From www.vedantu.com
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell. Fuel Cells Chemistry Notes a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. In the presence of two. fuel cells produce a voltage close. Fuel Cells Chemistry Notes.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Fuel Cells Chemistry Notes A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other. a fuel cell like this will continue to operate and produce electrical energy as long. Fuel Cells Chemistry Notes.