Fda Sterilization Standards . Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. This guidance replaces the 1987 industry guideline on sterile drug. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Manufacturing sterile drug and biological products using aseptic processing.
from mavink.com
Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. This guidance replaces the 1987 industry guideline on sterile drug. Manufacturing sterile drug and biological products using aseptic processing. Food and drug administration (fda) recently recognized the following sterilization standard and technical.
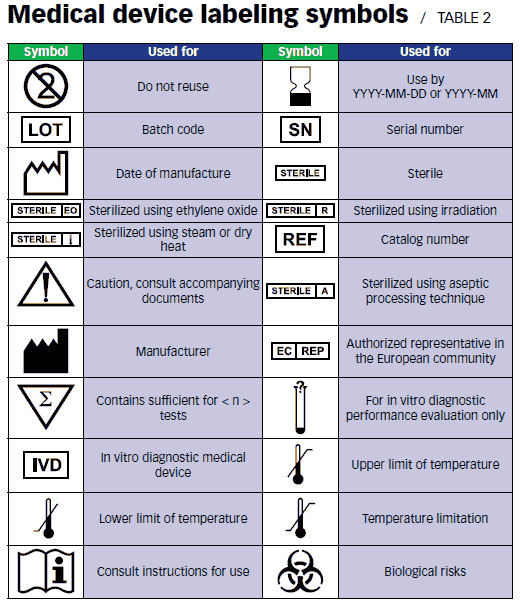
Medical Device Labeling Symbols
Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Manufacturing sterile drug and biological products using aseptic processing. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. This guidance replaces the 1987 industry guideline on sterile drug. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and technical.
From array.aami.org
FDA Recognizes AAMI Guidance on Pacemakers, Radiation Sterilization Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Medical devices are sterilized. Fda Sterilization Standards.
From shop.mhp-verlag.de
PDF eBook Legislation and international standards for sterilization Fda Sterilization Standards Food and drug administration (fda) recently recognized the following sterilization standard and technical. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug. Medical devices are sterilized. Fda Sterilization Standards.
From operonstrategist.com
FDA's Updated Sterilization Guidelines for Medical Device Operon Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug. Standard (iso 22441:2022). Fda Sterilization Standards.
From www.youtube.com
Difference Between STERILIZATION and SANITIZATION YouTube Fda Sterilization Standards This guidance replaces the 1987 industry guideline on sterile drug. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Manufacturing sterile drug and biological products using aseptic processing. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Standard (iso 22441:2022). Fda Sterilization Standards.
From www.aami.org
Industrial Sterilization for Medical Devices Virtual Training AAMI Fda Sterilization Standards Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Manufacturing sterile drug and biological products using aseptic processing. This guidance replaces. Fda Sterilization Standards.
From medicaldeviceacademy.com
How to select and help validate the best sterilization method? Fda Sterilization Standards Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug.. Fda Sterilization Standards.
From dimensionsofdentalhygiene.com
Ensure Infection Control Compliance With Chemical and Biological Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Amid a regulatory effort by the us environmental protection agency (epa) to. Fda Sterilization Standards.
From www.scribd.com
Guidance FDA STERILIZATION PDF PDF Federal Food Sterilization Fda Sterilization Standards Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. This guidance replaces the 1987 industry guideline on sterile drug. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and. Fda Sterilization Standards.
From emmainternational.com
FDA Sterilization Innovation Challenge EMMA International Fda Sterilization Standards Food and drug administration (fda) recently recognized the following sterilization standard and technical. Manufacturing sterile drug and biological products using aseptic processing. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. This guidance replaces the 1987 industry guideline on sterile drug. A list of recognized sterilization standards appears at fda's center for devices and. Fda Sterilization Standards.
From es.scribd.com
Sterilization ISO standards Sterilization (Microbiology) Materials Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. This guidance replaces the. Fda Sterilization Standards.
From www.slideshare.net
Sterilization Standards Update Strategies for Compliance Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Manufacturing sterile drug and biological products using aseptic processing. This guidance replaces the 1987 industry guideline on sterile drug. Food and drug administration (fda) recently. Fda Sterilization Standards.
From medicaldeviceacademy.com
510k Submission, Section 14Sterilization Validation and Shelflife Fda Sterilization Standards Food and drug administration (fda) recently recognized the following sterilization standard and technical. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. This guidance replaces the 1987 industry guideline on sterile drug. Medical devices are sterilized. Fda Sterilization Standards.
From hxrt666.en.made-in-china.com
CE, FDA, ISO Laboratory Standard Export Carton γ Irradiation Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. This guidance replaces the 1987 industry guideline on sterile drug. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on. Fda Sterilization Standards.
From mavink.com
Medical Device Labeling Symbols Fda Sterilization Standards Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020. Fda Sterilization Standards.
From www.steris-ast.com
Technical Tip Comparison of AAMI Methods for Dose Audits to Fda Sterilization Standards A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and technical. This guidance replaces the 1987 industry guideline on sterile drug. Manufacturing sterile drug and biological products using aseptic processing. Amid a regulatory effort by the us environmental protection agency. Fda Sterilization Standards.
From www.raps.org
FDA recognizes new medical device sterilization standards RAPS Fda Sterilization Standards A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Amid a regulatory effort by the us environmental protection. Fda Sterilization Standards.
From www.aplyon.com
Gamma Irradiation Sterilization Validation Procedure Fda Sterilization Standards A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. This guidance replaces the 1987 industry guideline on sterile drug. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene. Fda Sterilization Standards.
From www.scribd.com
Sterile Processing Checklist Sterilization (Microbiology) Chemistry Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Medical devices are sterilized in. Fda Sterilization Standards.
From www.presentationeze.com
Cleanroom Classification Information & current Best Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Food and drug administration (fda) recently recognized the following sterilization standard and technical. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the. Fda Sterilization Standards.
From www.alamy.com
Full set of medical device packaging symbols with warning information Fda Sterilization Standards This guidance replaces the 1987 industry guideline on sterile drug. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Standard (iso 22441:2022) reports (aami. Fda Sterilization Standards.
From compliancenavigator.bsigroup.com
Sterilization standardization moves into new areas Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Manufacturing sterile drug and biological products using aseptic processing. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide. Fda Sterilization Standards.
From www.yumpu.com
Standard Operating Procedures for Sterilization Services in Camps Fda Sterilization Standards Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Food and drug administration (fda) recently recognized the following sterilization standard and technical. A list of recognized sterilization standards appears at fda's center for devices and radiological health. Fda Sterilization Standards.
From www.raps.org
FDA recognizes new medical device sterilization standards RAPS Fda Sterilization Standards This guidance replaces the 1987 industry guideline on sterile drug. Food and drug administration (fda) recently recognized the following sterilization standard and technical. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide. Fda Sterilization Standards.
From stock.adobe.com
Full set of medical device packaging symbols with warning information Fda Sterilization Standards Manufacturing sterile drug and biological products using aseptic processing. Food and drug administration (fda) recently recognized the following sterilization standard and technical. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug. Fda Sterilization Standards.
From www.labcon.com
Labcon Sterility Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation,. Fda Sterilization Standards.
From onlinelibrary.wiley.com
Do Healthcare Professionals Comprehend Standardized Symbols Present on Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug. Manufacturing sterile drug and biological products using aseptic processing. Amid a regulatory effort by the us environmental. Fda Sterilization Standards.
From www.labcon.com
Labcon Sterility Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Food and drug administration (fda) recently recognized the following sterilization standard and technical. This guidance replaces the 1987 industry guideline on sterile drug. Manufacturing sterile drug and biological products using aseptic processing. A list of recognized sterilization standards appears at fda's center for devices and. Fda Sterilization Standards.
From www.alamy.com
Full vector set of sterilized and disinfectant symbols for medical Fda Sterilization Standards This guidance replaces the 1987 industry guideline on sterile drug. Manufacturing sterile drug and biological products using aseptic processing. Food and drug administration (fda) recently recognized the following sterilization standard and technical. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Medical devices are sterilized in a variety of ways including. Fda Sterilization Standards.
From oxymedz.com
Sterilization Of Medical Equipment OxyMed Fda Sterilization Standards Food and drug administration (fda) recently recognized the following sterilization standard and technical. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. This guidance replaces the 1987 industry guideline on sterile drug. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Manufacturing sterile. Fda Sterilization Standards.
From www.aami.org
Industrial Sterilization for Medical Devices Virtual Training AAMI Fda Sterilization Standards A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. This guidance replaces the 1987 industry guideline on sterile drug. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation,. Fda Sterilization Standards.
From materialrequirementform.blogspot.com
Material requirement form New drug approval process slideshare Fda Sterilization Standards Food and drug administration (fda) recently recognized the following sterilization standard and technical. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. This guidance replaces the 1987 industry guideline on sterile drug. A list of recognized sterilization standards appears at fda's center for devices and radiological health (cdrh's) web site. Amid a regulatory effort. Fda Sterilization Standards.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Sterilization Standards Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. Manufacturing sterile drug and biological products using aseptic processing. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. This guidance replaces the. Fda Sterilization Standards.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Sterilization Standards This guidance replaces the 1987 industry guideline on sterile drug. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Standard (iso 22441:2022) reports (aami tir 17:2017/ (r)2020 and aami tir104:2022) sterilization for medical devices. A list of. Fda Sterilization Standards.
From gxpcellators.com
Navigating FDA and EUGMP Requirements for Contamination Control A Fda Sterilization Standards Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its center for. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. Manufacturing sterile drug and biological products using aseptic. Fda Sterilization Standards.
From distilinfo.com
FDA Advances Medical Device Sterilization with Updated Standards Fda Sterilization Standards Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas,. This guidance replaces the 1987 industry guideline on sterile drug. Amid a regulatory effort by the us environmental protection agency (epa) to limit the use of ethylene oxide (eto), the food and drug administration (fda) on monday announced that its. Fda Sterilization Standards.