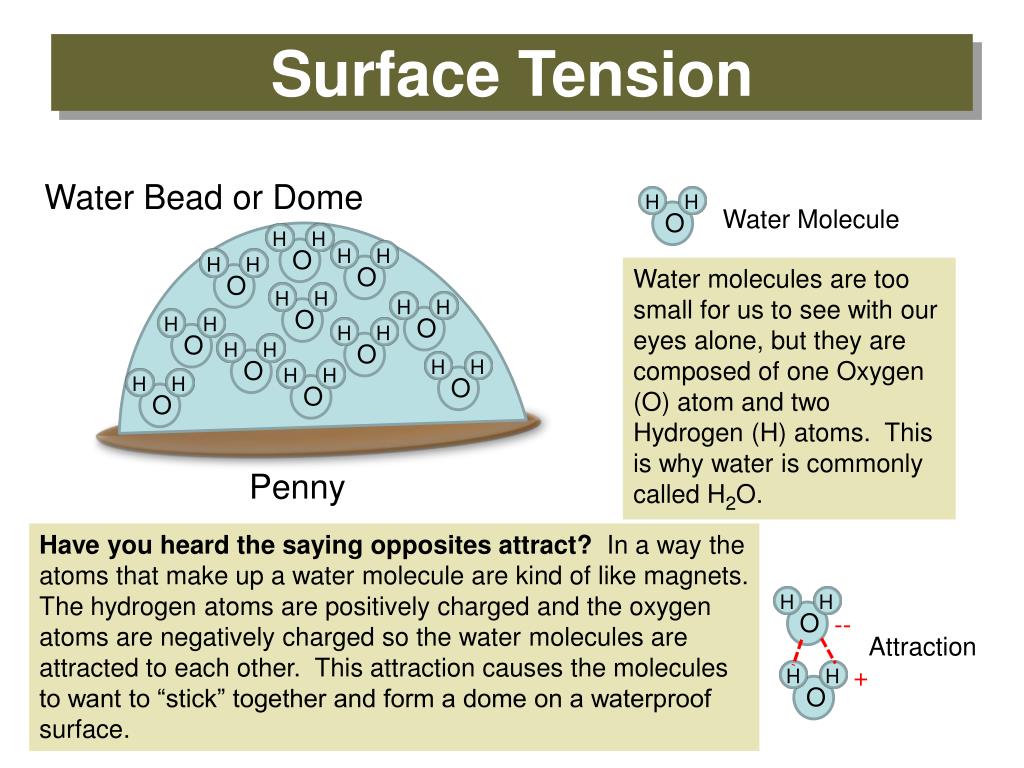
How Surface Tension Can Be Reduced . How does water purity affect surface tension? The surface tension of water decreases significantly with. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. The surface tension of water is 72 dynes/cm at 25°c. Surface tension results from a sharp change in the density between two adjoined. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. The presence of soluble particles in water may increase or decrease surface tension. The surface tension will decrease if. A few of them are as follows: Reduction of surface tension of water can be done in several ways.
from www.slideserve.com
The surface tension of water is 72 dynes/cm at 25°c. The surface tension will decrease if. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension results from a sharp change in the density between two adjoined. The presence of soluble particles in water may increase or decrease surface tension. A few of them are as follows: A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Reduction of surface tension of water can be done in several ways. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface.
PPT Surface Tension PowerPoint Presentation, free download ID3106425
How Surface Tension Can Be Reduced Reduction of surface tension of water can be done in several ways. The surface tension of water is 72 dynes/cm at 25°c. The surface tension will decrease if. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined. How does water purity affect surface tension? The surface tension of water decreases significantly with. The presence of soluble particles in water may increase or decrease surface tension. A few of them are as follows: It would take a force of 72 dynes to break a surface film of water 1 cm long. Reduction of surface tension of water can be done in several ways. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting.
From www.researchgate.net
Reduced surface tension is plotted as a function of temperature. The How Surface Tension Can Be Reduced Surface tension results from a sharp change in the density between two adjoined. A few of them are as follows: Reduction of surface tension of water can be done in several ways. The surface tension of water decreases significantly with. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. How Surface Tension Can Be Reduced.
From www.youtube.com
Why is surface tension important? YouTube How Surface Tension Can Be Reduced Surface tension results from a sharp change in the density between two adjoined. The surface tension will decrease if. How does water purity affect surface tension? The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Reduction of surface tension. How Surface Tension Can Be Reduced.
From www.youtube.com
What are the application of surface tension? YouTube How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. How does water purity affect surface tension? The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The surface tension of water decreases significantly. How Surface Tension Can Be Reduced.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface How Surface Tension Can Be Reduced Surface tension results from a sharp change in the density between two adjoined. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The surface tension of water decreases significantly with. Reduction of surface tension of water can be done in several ways. It would take a force of 72 dynes. How Surface Tension Can Be Reduced.
From www.multi-clean.com
MultiClean How to make a powerful cleaner from regular tap water How Surface Tension Can Be Reduced Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. The presence of soluble particles in water may increase or decrease surface tension. The surface tension is force per length and is. How Surface Tension Can Be Reduced.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state How Surface Tension Can Be Reduced The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The surface tension will decrease if. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does water purity affect surface tension? It would take a force of 72 dynes. How Surface Tension Can Be Reduced.
From slidetodoc.com
Lecture 14 Taken in part from Chapters 13 How Surface Tension Can Be Reduced It would take a force of 72 dynes to break a surface film of water 1 cm long. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. A few of them are as follows: The surface tension of water is 72 dynes/cm. How Surface Tension Can Be Reduced.
From www.pcimag.com
Solving Film Defects with Surface Tension Modifiers 20160401 PCI How Surface Tension Can Be Reduced Reduction of surface tension of water can be done in several ways. Surface tension results from a sharp change in the density between two adjoined. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is the energy, or work, required to increase the surface area of a liquid due. How Surface Tension Can Be Reduced.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The surface tension will decrease if. Reduction of surface tension of water can be. How Surface Tension Can Be Reduced.
From cynthianalibrary.org
Surface Tension Sink or Float? How Surface Tension Can Be Reduced The presence of soluble particles in water may increase or decrease surface tension. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A few of them are as follows: The surface tension of water decreases significantly with. It would take a force of 72 dynes to break a surface film. How Surface Tension Can Be Reduced.
From www.vrogue.co
Surface Tension Definition Units Epic Examples Effect vrogue.co How Surface Tension Can Be Reduced A few of them are as follows: How does water purity affect surface tension? The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined. Surface tension is the energy, or work, required to increase the surface area of. How Surface Tension Can Be Reduced.
From www.studocu.com
Surface Tension Surface Tension Introduction Surface tension can be How Surface Tension Can Be Reduced How does water purity affect surface tension? It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension results from a sharp change in the density between two adjoined. Reduction of surface tension of water can be done in several ways. A low surface tension can cause liquids to spread easily. How Surface Tension Can Be Reduced.
From ar.inspiredpencil.com
Surface Tension Water How Surface Tension Can Be Reduced The surface tension of water decreases significantly with. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. The surface tension of water is 72 dynes/cm at 25°c. A few of them are as follows: The surface tension will decrease if. Surface tension. How Surface Tension Can Be Reduced.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Surface Tension Can Be Reduced Reduction of surface tension of water can be done in several ways. A few of them are as follows: The surface tension will decrease if. The surface tension of water is 72 dynes/cm at 25°c. The presence of soluble particles in water may increase or decrease surface tension. How does water purity affect surface tension? A low surface tension can. How Surface Tension Can Be Reduced.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the. How Surface Tension Can Be Reduced.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Surface Tension Can Be Reduced Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does water purity affect surface tension? A few of them are as follows: The surface tension of water is 72 dynes/cm at 25°c. Surface tension results from a sharp change in the density between two adjoined. Reduction of surface. How Surface Tension Can Be Reduced.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension results from a sharp change in the density between two adjoined. The surface tension will decrease if. How does water purity affect surface tension? The surface tension is force per length. How Surface Tension Can Be Reduced.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension results from a sharp change in the density between two adjoined. The surface tension will decrease if. How does water purity affect surface tension? Reduction of surface tension of water can. How Surface Tension Can Be Reduced.
From www.youtube.com
Surface Tension YouTube How Surface Tension Can Be Reduced The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined. The surface tension will decrease if. The surface tension of water is 72 dynes/cm at 25°c. A low surface tension can cause liquids to spread easily on surfaces. How Surface Tension Can Be Reduced.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and How Surface Tension Can Be Reduced How does water purity affect surface tension? The presence of soluble particles in water may increase or decrease surface tension. Reduction of surface tension of water can be done in several ways. The surface tension will decrease if. The surface tension of water decreases significantly with. Surface tension is the energy, or work, required to increase the surface area of. How Surface Tension Can Be Reduced.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Surface Tension Can Be Reduced How does water purity affect surface tension? The surface tension of water is 72 dynes/cm at 25°c. The surface tension will decrease if. A few of them are as follows: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water decreases significantly with. The surface. How Surface Tension Can Be Reduced.
From bosstek.com
Dust Control Systems Cut Water Use in Drought BossTek How Surface Tension Can Be Reduced It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The presence of soluble particles in water may increase or decrease surface tension. The surface tension of water decreases significantly with. The surface. How Surface Tension Can Be Reduced.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension results from a sharp change in the density between two adjoined. The surface tension will decrease if. Reduction of surface tension of water can be done in several ways. How does. How Surface Tension Can Be Reduced.
From www.animalia-life.club
Surface Tension Of Water On A Leaf How Surface Tension Can Be Reduced The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. How does water purity affect surface tension? It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension will decrease if. The surface tension of water decreases significantly with. Surface tension. How Surface Tension Can Be Reduced.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Surface Tension Can Be Reduced A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The presence of soluble particles in water may increase or decrease surface tension. Reduction. How Surface Tension Can Be Reduced.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Surface Tension Can Be Reduced The surface tension of water is 72 dynes/cm at 25°c. The presence of soluble particles in water may increase or decrease surface tension. A few of them are as follows: The surface tension of water decreases significantly with. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is the. How Surface Tension Can Be Reduced.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences How Surface Tension Can Be Reduced Reduction of surface tension of water can be done in several ways. The surface tension of water is 72 dynes/cm at 25°c. The surface tension will decrease if. A few of them are as follows: The presence of soluble particles in water may increase or decrease surface tension. The surface tension is force per length and is measured by [n/m]. How Surface Tension Can Be Reduced.
From www.studypool.com
SOLUTION Surface tension of milk Studypool How Surface Tension Can Be Reduced The presence of soluble particles in water may increase or decrease surface tension. A few of them are as follows: It would take a force of 72 dynes to break a surface film of water 1 cm long. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension. How Surface Tension Can Be Reduced.
From www.biolinscientific.com
3 ways to measure surface tension How Surface Tension Can Be Reduced The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined. How does water purity affect surface tension? The surface tension will decrease if. Reduction of surface tension of water can be done in several ways. It would take. How Surface Tension Can Be Reduced.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b How Surface Tension Can Be Reduced The surface tension of water decreases significantly with. It would take a force of 72 dynes to break a surface film of water 1 cm long. Reduction of surface tension of water can be done in several ways. The surface tension will decrease if. Surface tension results from a sharp change in the density between two adjoined. The presence of. How Surface Tension Can Be Reduced.
From www.vrogue.co
Surface Tension Definition Examples Causes And Measur vrogue.co How Surface Tension Can Be Reduced Surface tension results from a sharp change in the density between two adjoined. Reduction of surface tension of water can be done in several ways. A few of them are as follows: It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with. The surface. How Surface Tension Can Be Reduced.
From readchemistry.com
Determination of Surface Tension Read Chemistry How Surface Tension Can Be Reduced Reduction of surface tension of water can be done in several ways. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension will decrease if. A few of them are as follows: Surface tension is the energy, or work, required to increase the surface area of a liquid due. How Surface Tension Can Be Reduced.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download How Surface Tension Can Be Reduced The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Reduction of surface tension of water can be done in several ways. It would take a force of 72 dynes to break a surface film of water 1 cm long. A few of them are as follows: The presence of soluble. How Surface Tension Can Be Reduced.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download How Surface Tension Can Be Reduced The surface tension of water decreases significantly with. A few of them are as follows: The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension will decrease if. The presence. How Surface Tension Can Be Reduced.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How Surface Tension Can Be Reduced Surface tension results from a sharp change in the density between two adjoined. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. How Surface Tension Can Be Reduced.