Iron Ii Oxide And Oxygen . Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron forms three different oxides: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can be formed from the. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron is very easily oxidized under alkaline conditions. Metal oxides act as bases. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Learn about what iron ii oxide is and how it is formed. Iron + dioxygen = iron(ii) oxide. This means they can neutralise an acid.
from www.slideserve.com
This means they can neutralise an acid. Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Iron(ii) oxide can be formed from the. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Metal oxides act as bases. Oxygen in the air oxidizes the iron(ii) hydroxide. Learn about what iron ii oxide is and how it is formed. Iron + dioxygen = iron(ii) oxide.
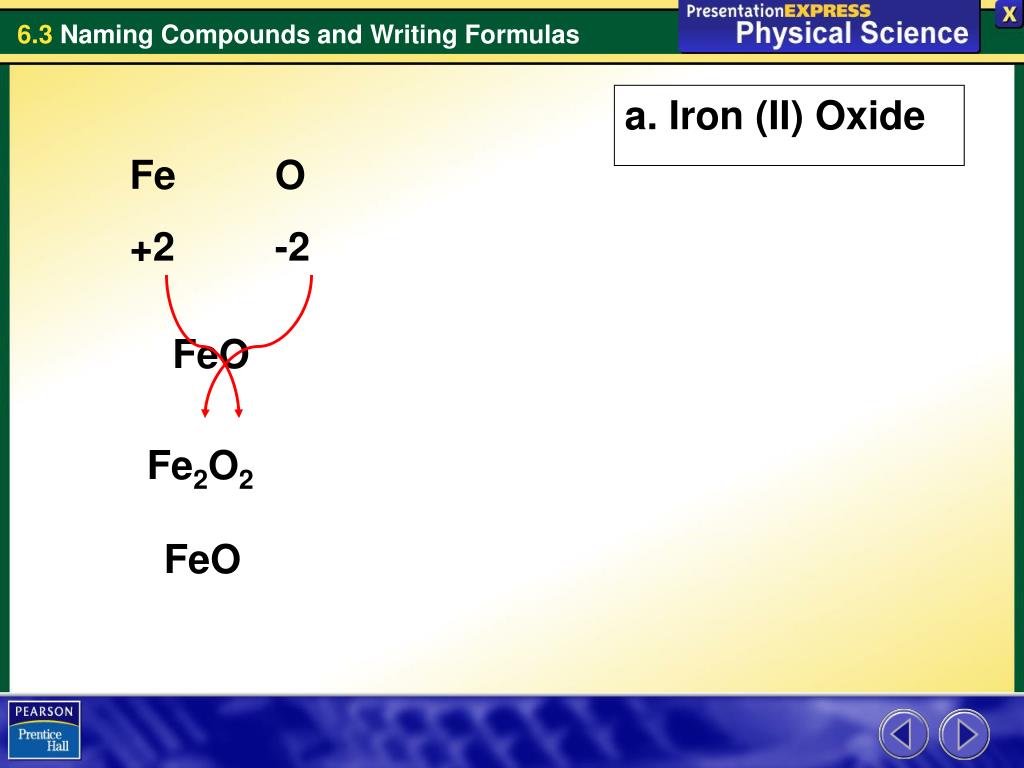
PPT What information do the name and formula of an ionic compound
Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Oxygen in the air oxidizes the iron(ii) hydroxide. Metal oxides act as bases. Iron + dioxygen = iron(ii) oxide. Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Learn about what iron ii oxide is and how it is formed. This means they can neutralise an acid. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can be formed from the. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation.
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron Ii Oxide And Oxygen Metal oxides act as bases. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide.. Iron Ii Oxide And Oxygen.
From www.geologypage.com
Discovery of new iron oxides points to large oxygen source inside the Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide, iron(iii). Iron Ii Oxide And Oxygen.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron forms three different oxides: Iron(ii) oxide can. Iron Ii Oxide And Oxygen.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron Ii Oxide And Oxygen This means they can neutralise an acid. Learn about what iron ii oxide is and how it is formed. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to.. Iron Ii Oxide And Oxygen.
From www.meritnation.com
Question 3 explain the formulae Ans Feo is iron (II) oxide Fe203 is Iron Ii Oxide And Oxygen Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron forms three different oxides: Iron + dioxygen = iron(ii) oxide. Metal oxides act as bases. This means they can neutralise an acid. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron is very easily oxidized under alkaline conditions. Learn about what iron ii oxide is. Iron Ii Oxide And Oxygen.
From mavink.com
Iron Oxygen Phase Diagram Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. This means they can neutralise an acid. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron forms three different oxides: Metal oxides act as bases. Iron + dioxygen. Iron Ii Oxide And Oxygen.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron Ii Oxide And Oxygen Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Oxygen in the air oxidizes the iron(ii) hydroxide. Iron is very easily oxidized under alkaline conditions.. Iron Ii Oxide And Oxygen.
From www.researchgate.net
Ironoxygen phase diagram [17]. Download Scientific Diagram Iron Ii Oxide And Oxygen Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron(ii) oxide can be formed from the. Iron + dioxygen = iron(ii) oxide. Iron forms three different oxides: Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron is very. Iron Ii Oxide And Oxygen.
From www.slideserve.com
PPT What information do the name and formula of an ionic compound Iron Ii Oxide And Oxygen Metal oxides act as bases. Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron + dioxygen = iron(ii) oxide. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can. Iron Ii Oxide And Oxygen.
From www.mdpi.com
Free FullText Diversity of Iron Oxides Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron(ii) oxide can be formed from the. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: This means they can neutralise an acid.. Iron Ii Oxide And Oxygen.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron Ii Oxide And Oxygen This means they can neutralise an acid. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron is very easily oxidized under alkaline conditions. Iron forms three different oxides: Oxygen in the air oxidizes the iron(ii) hydroxide. Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide can be formed from the. Understand the iron ii oxide. Iron Ii Oxide And Oxygen.
From www.researchgate.net
The DFTcalculated free energy of forming oxygen vacancies at a metal Iron Ii Oxide And Oxygen Iron is very easily oxidized under alkaline conditions. Iron forms three different oxides: Iron(ii) oxide can be formed from the. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can react with dilute mineral acids. Iron Ii Oxide And Oxygen.
From slideplayer.com
Ionic Compounds Naming ppt download Iron Ii Oxide And Oxygen Metal oxides act as bases. This means they can neutralise an acid. Iron is very easily oxidized under alkaline conditions. Iron(ii) oxide can be formed from the. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Oxygen in the air oxidizes the iron(ii) hydroxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one. Iron Ii Oxide And Oxygen.
From www.amertek.co.uk
Iron (II) Oxide (Black) / Ferrous Oxide FeO Good Grade Powder (Iron Iron Ii Oxide And Oxygen Iron forms three different oxides: Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide can be formed from the. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can react with dilute mineral acids according. Iron Ii Oxide And Oxygen.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Iron Ii Oxide And Oxygen Iron forms three different oxides: Metal oxides act as bases. Learn about what iron ii oxide is and how it is formed. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Understand the iron ii oxide formula, properties and uses, and see an iron ii. Iron Ii Oxide And Oxygen.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron Ii Oxide And Oxygen Iron is very easily oxidized under alkaline conditions. Learn about what iron ii oxide is and how it is formed. This means they can neutralise an acid. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron + dioxygen = iron(ii) oxide. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide. Iron Ii Oxide And Oxygen.
From www.youtube.com
How to write the formula for iron (II) oxide YouTube Iron Ii Oxide And Oxygen Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron is very easily oxidized under alkaline conditions. Iron forms three different oxides: Learn about what iron ii oxide is and how it is formed. This means they can neutralise an acid. Metal oxides act as bases. Iron(ii). Iron Ii Oxide And Oxygen.
From www.numerade.com
SOLVED 'In this laboratory experiment; two oxides of iron can form Iron Ii Oxide And Oxygen Learn about what iron ii oxide is and how it is formed. Iron is very easily oxidized under alkaline conditions. This means they can neutralise an acid. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can be formed from the. Iron(ii). Iron Ii Oxide And Oxygen.
From www.chegg.com
Solved Iron(II) oxide is produced by the rusting of Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron forms three different oxides: Learn about what iron ii oxide is and how it is formed. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 =. Iron Ii Oxide And Oxygen.
From www.toppr.com
Iron forms two oxides, in first oxide 56 grams of Iron is found to be Iron Ii Oxide And Oxygen Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Metal oxides act as bases. Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide can be formed from the. Fe + o2 = feo is a synthesis. Iron Ii Oxide And Oxygen.
From slideplayer.com
The Law of Conservation of Mass ppt download Iron Ii Oxide And Oxygen Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron(ii) oxide can be formed from the. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide.. Iron Ii Oxide And Oxygen.
From www.vrogue.co
What Are The Respective Formulas For Iron Ii Oxide An vrogue.co Iron Ii Oxide And Oxygen Oxygen in the air oxidizes the iron(ii) hydroxide. Iron is very easily oxidized under alkaline conditions. Iron + dioxygen = iron(ii) oxide. Iron forms three different oxides: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. This means they can neutralise an acid. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron(ii). Iron Ii Oxide And Oxygen.
From www.youtube.com
Lewis Structure of Iron (II) Oxide, FeO YouTube Iron Ii Oxide And Oxygen Learn about what iron ii oxide is and how it is formed. This means they can neutralise an acid. Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Metal oxides act as bases. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron(ii) oxide can be formed from the. Fe +. Iron Ii Oxide And Oxygen.
From www.pw.live
Iron II Oxide Formula Structure, Properties, Uses Iron Ii Oxide And Oxygen Metal oxides act as bases. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and. Iron Ii Oxide And Oxygen.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron Ii Oxide And Oxygen Iron(ii) oxide can be formed from the. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Learn about what iron ii oxide is and how it is formed. Iron forms three different oxides: Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron + dioxygen = iron(ii). Iron Ii Oxide And Oxygen.
From www.youtube.com
Iron(II) oxide YouTube Iron Ii Oxide And Oxygen Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron(ii) oxide can be formed from the. Iron + dioxygen = iron(ii) oxide. Iron forms three different oxides: Metal oxides act as bases. This means they can neutralise an acid. Iron is very easily. Iron Ii Oxide And Oxygen.
From en.wikipedia.org
Iron(II) oxide Wikipedia Iron Ii Oxide And Oxygen Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron is very easily oxidized under alkaline conditions. Iron forms three. Iron Ii Oxide And Oxygen.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The Iron Ii Oxide And Oxygen Iron + dioxygen = iron(ii) oxide. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. This means they can neutralise an acid. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Metal oxides act as bases. Iron forms three different oxides: Iron(ii) oxide can be formed from the. Oxygen in the air oxidizes. Iron Ii Oxide And Oxygen.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Iron Ii Oxide And Oxygen Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Metal oxides act as bases. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Learn about what iron ii oxide is and how it is formed. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron(ii) oxide can be. Iron Ii Oxide And Oxygen.
From www.youtube.com
How to Balance Fe + O2 + H2O = Fe2O3 . xH2O (Iron + Oxygen gas + Water Iron Ii Oxide And Oxygen Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Iron + dioxygen = iron(ii) oxide. Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe]. Iron Ii Oxide And Oxygen.
From www.mdpi.com
Free FullText Diversity of Iron Oxides Iron Ii Oxide And Oxygen Iron + dioxygen = iron(ii) oxide. This means they can neutralise an acid. Iron is very easily oxidized under alkaline conditions. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Learn. Iron Ii Oxide And Oxygen.
From www.slideserve.com
PPT Chemical Reactions and Equations PowerPoint Presentation, free Iron Ii Oxide And Oxygen Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron(ii) oxide, iron(iii) oxide, and iron(ii, iii) oxide. Learn about what iron ii oxide is and how it is formed. Iron is very easily oxidized under alkaline conditions. Iron forms three different oxides: Iron(ii) oxide can be formed from the. Metal oxides act. Iron Ii Oxide And Oxygen.
From www.echemi.com
Iron reacts with oxygen gas and iron (ii) oxide was produced. What are Iron Ii Oxide And Oxygen Iron forms three different oxides: Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Iron + dioxygen = iron(ii) oxide. Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide can be formed from the. Oxygen in the air oxidizes the iron(ii) hydroxide. Metal oxides act as bases.. Iron Ii Oxide And Oxygen.
From www.numerade.com
SOLVED Under certain conditions, the substances iron and oxygen Iron Ii Oxide And Oxygen Iron forms three different oxides: Iron is very easily oxidized under alkaline conditions. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Oxygen in the air oxidizes the iron(ii) hydroxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o 2] combine to. This. Iron Ii Oxide And Oxygen.
From www.researchgate.net
Phase diagram of stable iron oxide Download Scientific Diagram Iron Ii Oxide And Oxygen Learn about what iron ii oxide is and how it is formed. Iron(ii) oxide can react with dilute mineral acids according to the following general equation: Iron forms three different oxides: Understand the iron ii oxide formula, properties and uses, and see an iron ii oxide balanced equation. Fe + o2 = feo is a synthesis reaction where two moles. Iron Ii Oxide And Oxygen.