Heat Iron Reaction . Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Exothermic reactions change chemical energy into heat energy. A positive δh means that heat flows into a system from its. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. At each stage, students look for. Even alloys such as steel need. A negative \(δh\) means that heat flows from a system to its surroundings; Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly.
from www.vedantu.com
A positive δh means that heat flows into a system from its. A negative \(δh\) means that heat flows from a system to its surroundings; Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Even alloys such as steel need. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). At each stage, students look for.
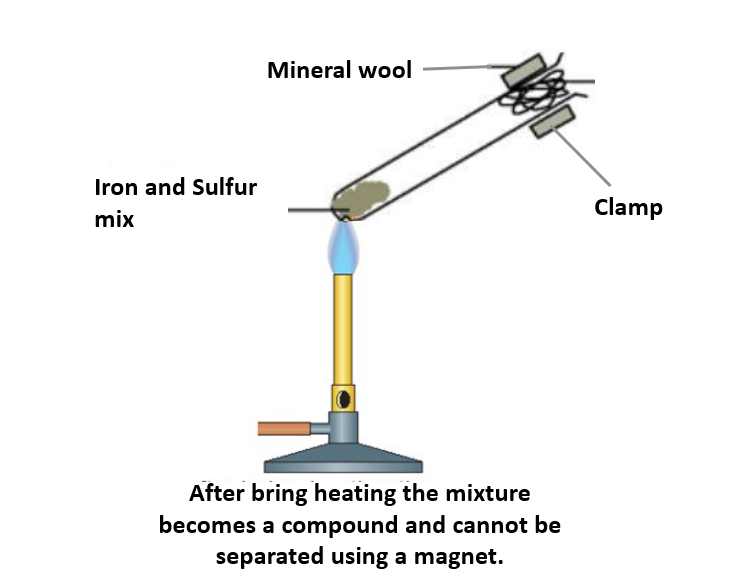
What is formed if a mixture of powdered iron and sulphur is heated in a
Heat Iron Reaction A negative \(δh\) means that heat flows from a system to its surroundings; Exothermic reactions change chemical energy into heat energy. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. At each stage, students look for. A negative \(δh\) means that heat flows from a system to its surroundings; Even alloys such as steel need. A positive δh means that heat flows into a system from its. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series.
From fractory.com
IronCarbon Phase Diagram Explained [with Graphs] Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. A positive δh means that heat flows into a system from its. The. Heat Iron Reaction.
From www.chegg.com
Solved Solid iron can be obtained by heating iron (III) Heat Iron Reaction Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). At each stage, students look for. A positive δh means that heat flows into a system from its. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. The salty water speeds. Heat Iron Reaction.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Exothermic reactions change chemical energy into heat energy. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Concentrated. Heat Iron Reaction.
From www.youtube.com
Action of Heat on Ferrous Sulphate Crystals Reaction Heat Iron Reaction Even alloys such as steel need. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. A negative \(δh\) means that heat flows from a system to its surroundings; In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine. Heat Iron Reaction.
From mammothmemory.net
Iron introduced to heat and steam turns to black iron oxide Heat Iron Reaction A positive δh means that heat flows into a system from its. Exothermic reactions change chemical energy into heat energy. At each stage, students look for. Even alloys such as steel need. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Pure iron reacts readily with oxygen and moisture in the environment. Heat Iron Reaction.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Heat Iron Reaction The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. A negative \(δh\) means that heat flows from a system to its surroundings; Exposing the iron to oxygen in. Heat Iron Reaction.
From www.chegg.com
Solved The reaction of iron with oxygen is very familiar. Heat Iron Reaction A negative \(δh\) means that heat flows from a system to its surroundings; Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. At each stage, students look for. The salty water speeds the reaction, while the filler moderates. Heat Iron Reaction.
From www.nuclear-power.com
Iron Specific Heat, Latent Heat of Fusion, Latent Heat of Heat Iron Reaction Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. A positive δh means that heat flows into a system from its. A negative \(δh\) means that heat flows from a system to its surroundings; Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). The salty water speeds the reaction, while the. Heat Iron Reaction.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation ID2132853 Heat Iron Reaction The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. Exothermic reactions change chemical energy into heat energy. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Exposing the iron to. Heat Iron Reaction.
From www.youtube.com
Iron(III) Thiocyanate Equilibrium // HSC Chemistry YouTube Heat Iron Reaction Even alloys such as steel need. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Exothermic reactions change chemical energy into heat energy. A positive δh means. Heat Iron Reaction.
From rstomar3114.blogspot.com
Rusty's Biozone CHEMICAL REACTIONS AND EQUATIONS Heat Iron Reaction Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. At each stage, students look for. Pure iron reacts readily with oxygen and moisture in the. Heat Iron Reaction.
From www.pinterest.com
REACTION OF IRON WITH SULPHUR The creator, Reactions, Sulphur Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. A positive δh means that heat flows into a. Heat Iron Reaction.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation, free download ID2132853 Heat Iron Reaction A positive δh means that heat flows into a system from its. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. Iron reacts with oxygen, o 2, forming. Heat Iron Reaction.
From www.youtube.com
The Effects of Heat on Matter Inquiry 6.1 Heating Sulfur YouTube Heat Iron Reaction A positive δh means that heat flows into a system from its. Exothermic reactions change chemical energy into heat energy. At each stage, students look for. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii).. Heat Iron Reaction.
From melscience.com
Characteristics of iron and its reaction with oxygen MEL Chemistry Heat Iron Reaction Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. A negative \(δh\) means that heat flows from a system to its surroundings; Even alloys such as steel need. Exothermic reactions change chemical energy into heat energy. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Concentrated nitric acid, hno 3, reacts on. Heat Iron Reaction.
From legendofsafety.com
🎉 Specific heat of iron. How to determine the specific heat capacity of Heat Iron Reaction At each stage, students look for. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. A negative \(δh\) means that heat flows from a system to its surroundings; The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure.. Heat Iron Reaction.
From www.numerade.com
If the combination of iron filings and sulfur in Question 25 is heated Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. A positive δh means that heat flows into a system from its. Even alloys such as steel need. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). A negative \(δh\) means that. Heat Iron Reaction.
From www.researchgate.net
The thermodynamic calculation of the reactions between iron oxides and Heat Iron Reaction The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. A negative \(δh\) means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from. Heat Iron Reaction.
From es.slideshare.net
Chemical Reactions Heat Iron Reaction Exothermic reactions change chemical energy into heat energy. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Even alloys such as steel need. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. The salty water speeds the reaction,. Heat Iron Reaction.
From www.metalworkingworldmagazine.com
How iron feels the heat Metal Working World Magazine Heat Iron Reaction At each stage, students look for. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. The salty water speeds the reaction, while the filler moderates it so. Heat Iron Reaction.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. At each stage, students look for. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. The salty water. Heat Iron Reaction.
From www.nagwa.com
Question Video Identifying the Reaction in a Blast Furnace That Helps Heat Iron Reaction Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. At each stage, students look for. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Concentrated nitric acid, hno 3, reacts on the surface of iron. Heat Iron Reaction.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Heat Iron Reaction At each stage, students look for. Even alloys such as steel need. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. A negative \(δh\) means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from its. Iron reacts with oxygen, o 2, forming fe. Heat Iron Reaction.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Heat Iron Reaction Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. A positive δh means that heat flows into a system from its. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. At each stage, students look. Heat Iron Reaction.
From sciencenotes.org
Exothermic Reactions Definition and Examples Heat Iron Reaction In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. Exothermic reactions change chemical energy into heat energy. Pure iron reacts readily with oxygen and moisture in the environment and. Heat Iron Reaction.
From www.youtube.com
Reactions of Iron Reactions Chemistry FuseSchool YouTube Heat Iron Reaction Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Concentrated nitric acid, hno 3, reacts on the surface of iron and passivates the surface. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. Exothermic reactions change chemical energy into heat energy. A negative \(δh\) means that heat. Heat Iron Reaction.
From tophat.com
OpenStax General Chemistry CH19 Transition Metals and Coordination Heat Iron Reaction The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. Even alloys such as steel need. In this experiment, students heat iron metal with the oxides of two other metals,. Heat Iron Reaction.
From www.youtube.com
The action of heat on Hydrated iron II sulphate YouTube Heat Iron Reaction At each stage, students look for. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). A negative \(δh\) means that heat flows from a system to its surroundings; Exothermic reactions change chemical energy into heat energy. Even alloys such as. Heat Iron Reaction.
From www.youtube.com
Science Experiment Rate of Conductivity of Heat Iron & Aluminium Heat Iron Reaction Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. At each stage, students look for. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. Even alloys such as steel need. Concentrated nitric acid, hno 3, reacts on the surface of iron and. Heat Iron Reaction.
From www.researchgate.net
Heat of reactions versus temperature for iron oxide reduction by Heat Iron Reaction Exothermic reactions change chemical energy into heat energy. A positive δh means that heat flows into a system from its. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. At each stage, students look for. Exposing the iron to oxygen in air makes. Heat Iron Reaction.
From www.chegg.com
Solved Determine the heat of reaction for the oxidation of Heat Iron Reaction Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. The salty water speeds the reaction, while the filler moderates it so it does not get too hot or end too quickly. A negative \(δh\) means that heat flows from a system to its. Heat Iron Reaction.
From yugajyoti.blogspot.com
ALL IN ONE REACTION INSIDE THE BLAST FURNACE Heat Iron Reaction Even alloys such as steel need. Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). A positive δh means that heat flows into a system from its. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. At each stage, students look. Heat Iron Reaction.
From www.youtube.com
Iron Iron Carbide Equilibrium Diagram or fefe3c phase diagram All Heat Iron Reaction Iron reacts with oxygen, o 2, forming fe (ii) and fe (iii). Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium,. Heat Iron Reaction.
From www.vedantu.com
What is formed if a mixture of powdered iron and sulphur is heated in a Heat Iron Reaction The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. At each stage, students look for. Exothermic reactions change chemical energy into heat energy. A positive δh means that heat flows into a system from its. Pure iron reacts readily with oxygen and moisture. Heat Iron Reaction.
From www.vedantu.com
Draw a neat diagram of the blast furnace used in the extraction of iron Heat Iron Reaction Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. Exposing the iron to oxygen in air makes iron oxide (fe 2 o 3 ) or rust. A negative \(δh\) means that heat flows from a system to its surroundings; Exothermic reactions change chemical energy into heat energy. Iron reacts with oxygen, o 2, forming fe. Heat Iron Reaction.