Medical Device Harms List . International standard bs en iso 14971 [1] was developed to. A failure mode may be. What is risk management for medical devices? Risk management for medical devices helps manufacturers identify potential hazards and assess the. Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device.
from naturalon.com
A failure mode may be. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed to. Risk management for medical devices helps manufacturers identify potential hazards and assess the. Gnosis and treatment, they should be protected from risks that could further impact their health.
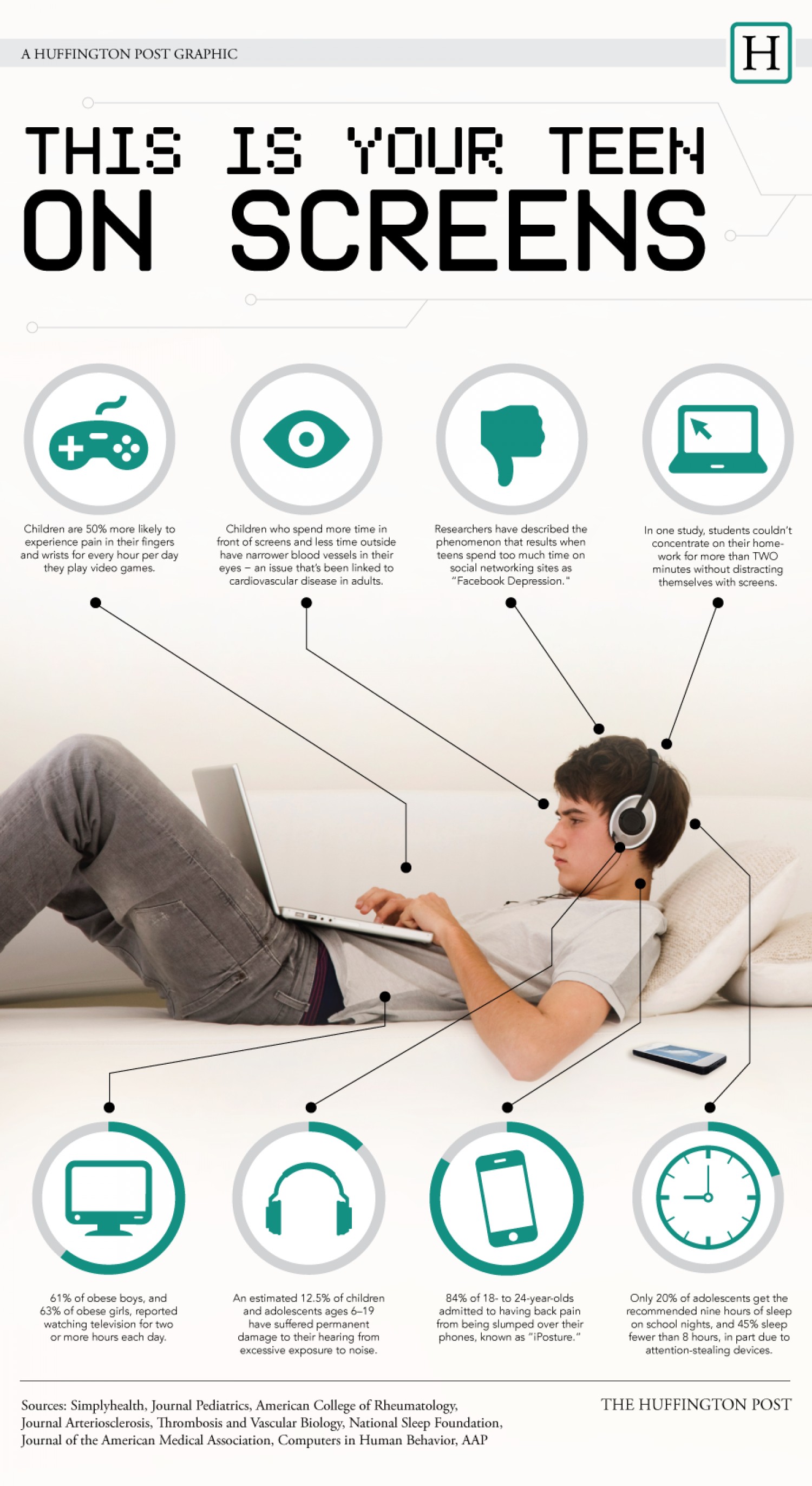
8 Ways Technology Affects Health Infographic
Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. A failure mode may be. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? International standard bs en iso 14971 [1] was developed to. Gnosis and treatment, they should be protected from risks that could further impact their health.
From www.bemedwise.org
The Dangers of Medicine Abuse BeMedwise BeMedwise Medical Device Harms List International standard bs en iso 14971 [1] was developed to. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Gnosis and treatment, they should be protected from risks that could further impact their health. Risk management. Medical Device Harms List.
From elizabeth-blakelock.medium.com
Common harms across essential services by Elizabeth Blakelock Medium Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Gnosis and treatment, they should be protected from risks that could further impact their health. A failure mode may be. What is risk management for medical devices? International standard bs en iso 14971 [1]. Medical Device Harms List.
From www.slideserve.com
PPT Awareness of Risks and Responsibilities PowerPoint Presentation Medical Device Harms List What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. A failure mode may. Medical Device Harms List.
From jamanetwork.com
Physician Understanding of Harms and Benefits of Common Medical Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their. Medical Device Harms List.
From medicaldevicehq.com
FMEA vs ISO 14971 Medical Device HQ 1 Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. A failure mode may be. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Gnosis and treatment, they should be. Medical Device Harms List.
From www.tampabay.com
Hidden FDA reports detail harm caused by scores of medical devices Medical Device Harms List What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. A failure mode may be. Risk management for medical devices helps manufacturers. Medical Device Harms List.
From www.todaysmedicaldevelopments.com
MEDICAL DEVICE COUNTERFEITING HARMS MANY Today's Medical Developments Medical Device Harms List A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed. Medical Device Harms List.
From www.semanticscholar.org
Case study — Risk management for medical devices (based on ISO 14971 Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. A failure mode may be. Gnosis and treatment, they should be protected from risks that could further impact their health. International. Medical Device Harms List.
From www.researchgate.net
Effect of adjunctive devices on intermediate health and harms Medical Device Harms List A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under. Medical Device Harms List.
From www.slideserve.com
PPT Medical Device Reporting and Tracking PowerPoint Presentation Medical Device Harms List What is risk management for medical devices? A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed. Medical Device Harms List.
From www.researchgate.net
Flow diagram of the hazard analysis process followed. The steps of the Medical Device Harms List A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed. Medical Device Harms List.
From sciencebasedmedicine.org
Less benefit, more risk. Our assumptions about health treatments are Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. What is risk management for medical. Medical Device Harms List.
From www.youtube.com
Managing and Tracing Risk Controls in Medical Device Development YouTube Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. International standard bs en iso 14971 [1] was developed to. A failure mode may be. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a. Medical Device Harms List.
From www.healthcarevaluehub.org
Interactive Medical Harm Infographic Altarum Healthcare Value Hub Medical Device Harms List International standard bs en iso 14971 [1] was developed to. Risk management for medical devices helps manufacturers identify potential hazards and assess the. Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of. Medical Device Harms List.
From jamanetwork.com
Deciphering Harm Measurement Health Care Safety JAMA The JAMA Network Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Gnosis and treatment, they should be protected from risks that could further impact their health. What is risk management for medical. Medical Device Harms List.
From spmscience.blog.onlinetuition.com.my
2.8 Effects of Drug Abuse on Health SPM Science Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? International standard bs en iso 14971 [1] was developed to. Gnosis and treatment, they. Medical Device Harms List.
From www.pinterest.com
A printable medication side effect tracker premium template is a Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed to. Gnosis and treatment, they should be protected from risks that could further impact their health. What is risk management for medical devices? Risk management. Medical Device Harms List.
From www.re-check.ch
Improving the detection, analysis, and reporting of harms in medicines Medical Device Harms List A failure mode may be. International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical. Medical Device Harms List.
From www.canada.ca
Opioidrelated Harms in Canada Integrating Emergency Medical Service Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them. Medical Device Harms List.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Harms List What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. International standard bs en iso 14971 [1] was developed to. Gnosis and treatment, they should be protected from risks that could further impact their health. A failure. Medical Device Harms List.
From pharmaceutical-journal.com
Medication errors where do they happen? The Pharmaceutical Journal Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential. Medical Device Harms List.
From nam.edu
Primary, Secondary, and Tertiary Prevention of Substance Use Disorders Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their health. A failure mode may be. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the. Medical Device Harms List.
From www.claritychi.com
What to Include in a Harm Reduction Kit Clarity Clinic Medical Device Harms List It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. A failure mode may be. What is risk management for medical. Medical Device Harms List.
From www.healthcarevaluehub.org
Interactive Consumer Harm Infographic Altarum Healthcare Value Hub Medical Device Harms List International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. Gnosis and treatment, they should be. Medical Device Harms List.
From www.medtecheurope.org
Page 37 of 45 Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. What is risk management for medical devices? Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. A failure mode may be. It is helpful to make a master hazards list under. Medical Device Harms List.
From www.algonquincollege.com
Harm Reduction as Best Practice Umbrella Project Medical Device Harms List What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their health. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. A failure mode may be. It is helpful to make a master hazards list under. Medical Device Harms List.
From www.druganddevicewatch.com
How Does Vaping Affect Your Body? Drug And Device Watch Medical Device Harms List What is risk management for medical devices? International standard bs en iso 14971 [1] was developed to. A failure mode may be. Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a. Medical Device Harms List.
From www.canada.ca
Drug and medical device highlights 2020 Medical devices Canada.ca Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. A failure mode may be. Risk management for medical devices helps manufacturers identify potential hazards and assess the. International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them. Medical Device Harms List.
From www.firstsafetysigns.co.uk
COSHH Know Your Dangerous Substances Poster First Safety Signs Medical Device Harms List What is risk management for medical devices? Risk management for medical devices helps manufacturers identify potential hazards and assess the. A failure mode may be. International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical. Medical Device Harms List.
From naturalon.com
8 Ways Technology Affects Health Infographic Medical Device Harms List Gnosis and treatment, they should be protected from risks that could further impact their health. What is risk management for medical devices? It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess. Medical Device Harms List.
From g-i-n.net
Presenting treatment options and communicating their risks and harms in Medical Device Harms List International standard bs en iso 14971 [1] was developed to. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? Gnosis and treatment, they. Medical Device Harms List.
From www.researchgate.net
Smart devices that may pose harms identified in systematic review Medical Device Harms List A failure mode may be. International standard bs en iso 14971 [1] was developed to. What is risk management for medical devices? Gnosis and treatment, they should be protected from risks that could further impact their health. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a. Medical Device Harms List.
From mpe-inc.com
Harms and Probabilities of Occurrence and Severity for Your Medical Device Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. A failure mode may be. International standard bs en iso 14971 [1] was developed to. Gnosis and treatment, they should be. Medical Device Harms List.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Harms List Risk management for medical devices helps manufacturers identify potential hazards and assess the. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Gnosis and treatment, they should be protected from risks that could further impact their health. What is risk management for medical. Medical Device Harms List.
From www.aligned.ch
The IMDRF terminologies a common risk language Medical Device Harms List A failure mode may be. It is helpful to make a master hazards list under different categories so you can evaluate them holistically within the scope of a given medical device. Risk management for medical devices helps manufacturers identify potential hazards and assess the. What is risk management for medical devices? Gnosis and treatment, they should be protected from risks. Medical Device Harms List.