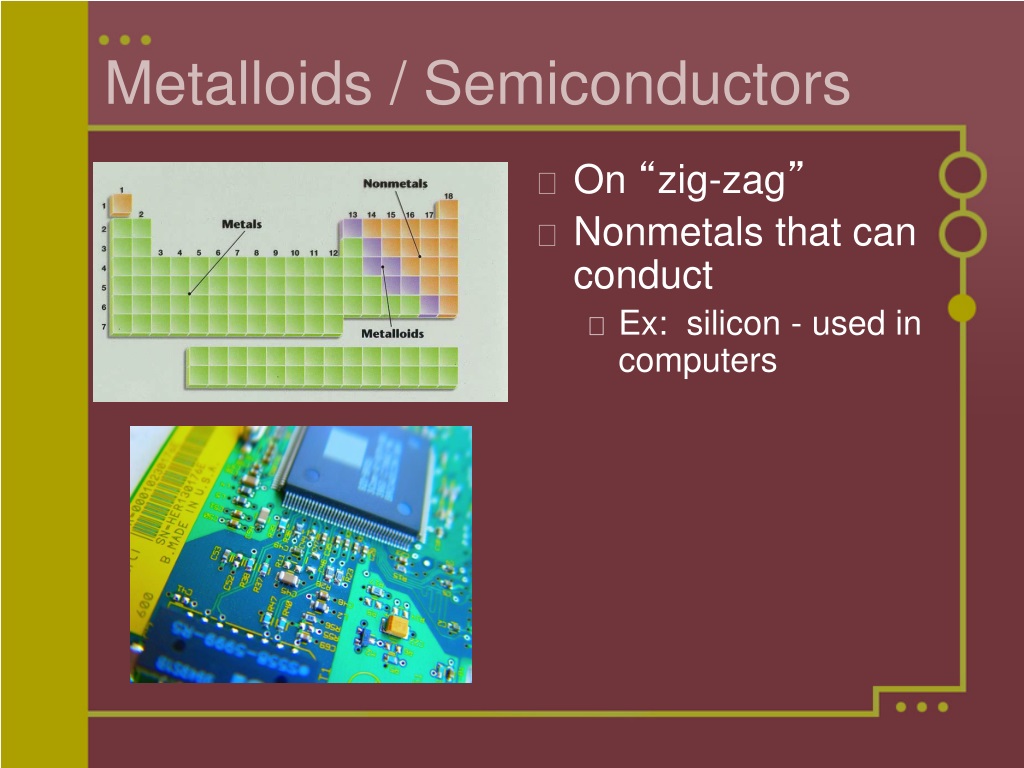
Metalloids Are Used In Semiconductors Because . You’ll also learn why metalloids and their compounds are used. Let’s find out what metalloids are and how they conduct electricity. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Unlike metals, they are neither malleable nor ductile. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Although they do not readily. Study with quizlet and memorize flashcards. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A nonmetal is used because it is a. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties.
from www.slideserve.com
You’ll also learn why metalloids and their compounds are used. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Unlike metals, they are neither malleable nor ductile. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Study with quizlet and memorize flashcards. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Let’s find out what metalloids are and how they conduct electricity. A nonmetal is used because it is a.
PPT Periodic Table Groups PowerPoint Presentation, free download ID9669115
Metalloids Are Used In Semiconductors Because Study with quizlet and memorize flashcards. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. A nonmetal is used because it is a. Although they do not readily. Study with quizlet and memorize flashcards. You’ll also learn why metalloids and their compounds are used. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Let’s find out what metalloids are and how they conduct electricity. Unlike metals, they are neither malleable nor ductile. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons.
From www.slideserve.com
PPT Periodic Table Groups PowerPoint Presentation, free download ID9669115 Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. You’ll also learn why metalloids and their compounds are used. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Let’s find out what metalloids are and how they conduct electricity. Unlike metals, they are neither malleable nor ductile. They are semiconductors because their electrons are more tightly. Metalloids Are Used In Semiconductors Because.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Metalloids Are Used In Semiconductors Because Although they do not readily. A nonmetal is used because it is a. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. You’ll also learn why metalloids and their compounds are used. Let’s find out what metalloids are and how they conduct electricity. Metalloids are. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Properties of Metals, Nonmetals, and Metalloids ppt download Metalloids Are Used In Semiconductors Because Let’s find out what metalloids are and how they conduct electricity. You’ll also learn why metalloids and their compounds are used. Unlike metals, they are neither malleable nor ductile. Although they do not readily. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A nonmetal is used because it is a.. Metalloids Are Used In Semiconductors Because.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are Used In Semiconductors Because They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Although they do not readily. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. A metalloid is used because it is a semiconductor and can become. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Lesson 7 October 5th, 2010 The Elements. ppt download Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. You’ll also learn why metalloids and their compounds are used. A nonmetal is used because it is a. Study with quizlet and memorize flashcards. Let’s find out what metalloids. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Table of Contents Book K Ch. 3 Sec. 1 Pg ppt download Metalloids Are Used In Semiconductors Because Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. You’ll also learn why metalloids and their compounds are used. Although they do not readily. Study with quizlet and memorize flashcards. A nonmetal is used because it is a. Let’s find. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Metalloids Are Used In Semiconductors Because Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Let’s find out what metalloids are and how they conduct electricity. A nonmetal is used because it is a. A phosphorus doped silicon. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Metals. ppt download Metalloids Are Used In Semiconductors Because You’ll also learn why metalloids and their compounds are used. Although they do not readily. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is used because it is a. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Lesson 7 October 5th, 2010 The Elements. ppt download Metalloids Are Used In Semiconductors Because A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. A nonmetal is used because it is a. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Study with quizlet and memorize flashcards.. Metalloids Are Used In Semiconductors Because.
From www.slideserve.com
PPT 11 th grade Physical Science Chapter 4 Notes PowerPoint Presentation ID1588495 Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Study with quizlet and memorize flashcards. A nonmetal is used because it is a. A metalloid is used because it is a semiconductor and. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Metalloids Lesson 3, pages ppt download Metalloids Are Used In Semiconductors Because A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Unlike metals, they are neither malleable nor ductile. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Let’s find out what metalloids are and how they conduct electricity. A nonmetal is. Metalloids Are Used In Semiconductors Because.
From www.slideserve.com
PPT The periodic table PowerPoint Presentation, free download ID1590027 Metalloids Are Used In Semiconductors Because Unlike metals, they are neither malleable nor ductile. Although they do not readily. Let’s find out what metalloids are and how they conduct electricity. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. They are semiconductors because their electrons are more tightly bound to their. Metalloids Are Used In Semiconductors Because.
From www.sliderbase.com
Element Classes Presentation Chemistry Metalloids Are Used In Semiconductors Because A nonmetal is used because it is a. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Although they do. Metalloids Are Used In Semiconductors Because.
From rillysmart.weebly.com
Periodic table definition Metalloid Semiconductor definition chemistry rillysmart Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Although they do not readily. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Study with quizlet and memorize flashcards. A metalloid is used because it is a semiconductor and can become more. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Section 4 The Periodic Table ppt download Metalloids Are Used In Semiconductors Because Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Although they do not readily. You’ll also learn why metalloids and their compounds are used. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical. Metalloids Are Used In Semiconductors Because.
From www.haikudeck.com
ELEMENTS by Michael Donoho Metalloids Are Used In Semiconductors Because A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Unlike metals, they are neither malleable nor ductile. Study with quizlet and memorize flashcards. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Although they do not readily. Most metalloids have a shiny,. Metalloids Are Used In Semiconductors Because.
From inquivixtech.com
Why Metalloids Are Useful As Semiconductors In Modern Electronics Inquivix Technologies Metalloids Are Used In Semiconductors Because A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing. Metalloids Are Used In Semiconductors Because.
From inquivixtech.com
Why Metalloids Are Useful As Semiconductors In Modern Electronics Inquivix Technologies Metalloids Are Used In Semiconductors Because Although they do not readily. Unlike metals, they are neither malleable nor ductile. Let’s find out what metalloids are and how they conduct electricity. A nonmetal is used because it is a. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties.. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Nonmetals and Metalloids ppt download Metalloids Are Used In Semiconductors Because Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Study with quizlet and memorize flashcards. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Unlike metals, they are neither malleable nor. Metalloids Are Used In Semiconductors Because.
From www.slideserve.com
PPT Atoms and the Periodic Table PowerPoint Presentation, free download ID5230194 Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Let’s find out what metalloids are and how they conduct electricity. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. You’ll also learn why metalloids and their compounds are used. A metalloid is used because it is a semiconductor and can become more conductive when more light. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Metalloids Are Used In Semiconductors Because Let’s find out what metalloids are and how they conduct electricity. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Unlike metals, they are neither malleable nor ductile. A key feature of a metal is its high. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Nonmetals and Metalloids ppt download Metalloids Are Used In Semiconductors Because Study with quizlet and memorize flashcards. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. A key feature of a metal is its high electrical conductivity, σ, but more importantly its. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Periodic Table SPW 234 Chapter ppt download Metalloids Are Used In Semiconductors Because Although they do not readily. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. A nonmetal is used because. Metalloids Are Used In Semiconductors Because.
From slidetodoc.com
Chapter 10 The Periodic Table Lesson 1 Using Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Let’s find out what metalloids are and how they conduct electricity. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
The Periodic Table of the Elements ppt download Metalloids Are Used In Semiconductors Because Let’s find out what metalloids are and how they conduct electricity. You’ll also learn why metalloids and their compounds are used. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. They are semiconductors because their electrons are more tightly bound to their nuclei than are. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
The Periodic Table. ppt download Metalloids Are Used In Semiconductors Because Let’s find out what metalloids are and how they conduct electricity. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. You’ll also learn why metalloids and their compounds are used. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it.. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Chapter 2 Atoms, Molecules, and Ions ppt download Metalloids Are Used In Semiconductors Because Unlike metals, they are neither malleable nor ductile. Although they do not readily. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. They are semiconductors because their electrons are more tightly bound to their nuclei. Metalloids Are Used In Semiconductors Because.
From www.slideserve.com
PPT Metalloids PowerPoint Presentation, free download ID1587638 Metalloids Are Used In Semiconductors Because You’ll also learn why metalloids and their compounds are used. A nonmetal is used because it is a. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Although they do not readily. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A key feature of a metal is its high. Metalloids Are Used In Semiconductors Because.
From www.chemistrylearner.com
Metalloids Chemistry Learner Metalloids Are Used In Semiconductors Because Although they do not readily. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of. Metalloids Are Used In Semiconductors Because.
From inquivixtech.com
Why Metalloids Are Useful As Semiconductors In Modern Electronics Inquivix Technologies Metalloids Are Used In Semiconductors Because A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. You’ll also learn why metalloids and their compounds are used. Most metalloids have a shiny, metallic appearance. Metalloids Are Used In Semiconductors Because.
From inquivixtech.com
Why Metalloids Are Useful As Semiconductors In Modern Electronics Inquivix Technologies Metalloids Are Used In Semiconductors Because Study with quizlet and memorize flashcards. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. A phosphorus. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
FÍSICA Y QUÍMICA 3º DE E.S.O. ppt download Metalloids Are Used In Semiconductors Because You’ll also learn why metalloids and their compounds are used. Let’s find out what metalloids are and how they conduct electricity. Although they do not readily. Unlike metals, they are neither malleable nor ductile. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. They are semiconductors because their electrons are more tightly bound to their nuclei than are those. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
The Periodic Table Metals, Metalloids, and Nonmetals. ppt download Metalloids Are Used In Semiconductors Because Let’s find out what metalloids are and how they conduct electricity. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Although they do not readily. A key feature of a metal is its high electrical conductivity, σ, but more importantly its electrical conductivity increases with decreasing temperature, σ ∝. Study with. Metalloids Are Used In Semiconductors Because.
From slideplayer.com
Mapping the Periodic Table ppt download Metalloids Are Used In Semiconductors Because A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Although they do not readily. Unlike metals, they are neither malleable nor ductile. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. You’ll also learn why metalloids and their compounds are used. A key feature of a metal. Metalloids Are Used In Semiconductors Because.
From www.slideserve.com
PPT Unit 1 PowerPoint Presentation, free download ID1587582 Metalloids Are Used In Semiconductors Because A metalloid is used because it is a semiconductor and can become more conductive when more light shines on it. Metalloids are brittle, lustrous metallic elements that exhibit semiconductive properties. Unlike metals, they are neither malleable nor ductile. A phosphorus doped silicon is an _____________ semiconductor because of extra electrons. Most metalloids have a shiny, metallic appearance but are brittle,. Metalloids Are Used In Semiconductors Because.