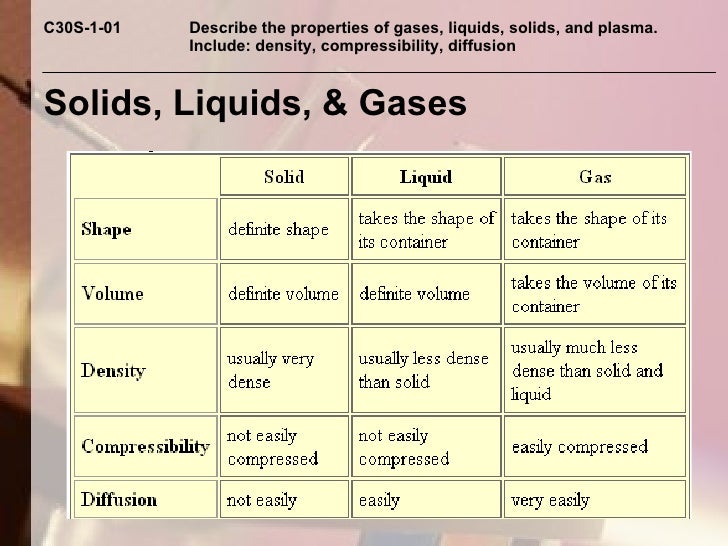
Solid Liquid Compressibility . Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Gases are highly compressible compared to solids and liquids due to the larger. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Explore the factors that affect the. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids.
from www.slideshare.net
Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Explore the factors that affect the. Gases are highly compressible compared to solids and liquids due to the larger. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and.
Unit 1 Notes
Solid Liquid Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Explore the factors that affect the. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Gases are highly compressible compared to solids and liquids due to the larger. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume.
From brainly.in
How do you differentiate between a solid and a liquid base on Solid Liquid Compressibility Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn about. Solid Liquid Compressibility.
From www.numerade.com
SOLVEDWhy are gases so much more compressible than solids or liquids? Solid Liquid Compressibility Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Gases are highly compressible compared to solids and liquids due to the larger. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Explore the factors that affect the. Learn how to define and calculate. Solid Liquid Compressibility.
From www.pw.live
Class 9 chemistry Notes Matter is our surrounding STATES OF MATTER Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Gases are highly compressible compared to solids and liquids. Solid Liquid Compressibility.
From www.youtube.com
compressibility of solids, liquids and gases YouTube Solid Liquid Compressibility Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Explore the factors that affect the. Learn the definition and examples of solids, liquids and gases, and how. Solid Liquid Compressibility.
From slideplayer.com
Solids Chapter 10 Section ppt download Solid Liquid Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how the kinetic molecular theory of gases can be applied to. Solid Liquid Compressibility.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Solid Liquid Compressibility Explore the factors that affect the. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn the definition and examples of solids, liquids and gases, and. Solid Liquid Compressibility.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID1797453 Solid Liquid Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Explore the factors that affect the. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how liquids can be compressed by. Solid Liquid Compressibility.
From www.youtube.com
Compressibility of solid, liquid and gas YouTube Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how liquids can be compressed by pressure and temperature, and how their. Solid Liquid Compressibility.
From www.slideserve.com
PPT Matter & Energy PowerPoint Presentation, free download ID5368269 Solid Liquid Compressibility Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Explore the factors that affect the. Gases are highly compressible compared to solids and liquids due to. Solid Liquid Compressibility.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID8890944 Solid Liquid Compressibility Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids.. Solid Liquid Compressibility.
From dxojeueds.blob.core.windows.net
Solids Liquids And Gases Third Grade at Ann Trotter blog Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Gases are highly compressible compared to solids and liquids due to the larger. Learn about the compressibility of solids, liquids and gases, and how it relates to. Solid Liquid Compressibility.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Solid Liquid Compressibility Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn about the compressibility of solids, liquids and gases,. Solid Liquid Compressibility.
From brainly.in
III. Complete the following table. 10. Property Solid Liquid Gas Shape Solid Liquid Compressibility Explore the factors that affect the. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how. Solid Liquid Compressibility.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid Compressibility Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Compressibility. Solid Liquid Compressibility.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid Compressibility Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how liquids can be compressed by pressure and. Solid Liquid Compressibility.
From joilgqxpm.blob.core.windows.net
Solids Liquids And Gases Ks2 at Eric Lee blog Solid Liquid Compressibility Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Gases are highly compressible compared to solids and liquids due to the larger. Learn how particles are arranged and move in solids, liquids and gases using the. Solid Liquid Compressibility.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Solid Liquid Compressibility Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Explore the factors that affect the. Learn how particles are arranged and move in. Solid Liquid Compressibility.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn how. Solid Liquid Compressibility.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Compressibility Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids. Solid Liquid Compressibility.
From byjus.com
Which of the following shows Highest interpartical spaces Highest Solid Liquid Compressibility Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter.. Solid Liquid Compressibility.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how the kinetic molecular theory of gases can be. Solid Liquid Compressibility.
From coffeytrust.blogspot.com
Compressibility Definition in Fluid Mechanics Coffeytrust Solid Liquid Compressibility Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Gases are highly compressible compared to solids and liquids due to the larger. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Explore the factors that affect the. Learn how particles are arranged and move. Solid Liquid Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid Compressibility Explore the factors that affect the. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Gases are highly. Solid Liquid Compressibility.
From slideplayer.com
Chemistry Notes Phases of Matter ppt download Solid Liquid Compressibility Explore the factors that affect the. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Learn how. Solid Liquid Compressibility.
From www.slideshare.net
(Science) Matter Solid Liquid Compressibility Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how to define and calculate the isothermal. Solid Liquid Compressibility.
From www.slideshare.net
Unit 1 Notes Solid Liquid Compressibility Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Explore the factors that affect the. Learn how liquids can be compressed by pressure and temperature, and how their compressibility affects their speed of. Compressibility is. Solid Liquid Compressibility.
From www.slideshare.net
Compressibility of solids , liquids and gases Solid Liquid Compressibility Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Explore the factors that affect the. Learn how liquids can be compressed by pressure and temperature, and. Solid Liquid Compressibility.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free Solid Liquid Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Explore the factors that affect the. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Compressibility is the measure of reducing the volume. Solid Liquid Compressibility.
From brainly.in
hello describe the activity to compare the compressibility of solid Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Gases. Solid Liquid Compressibility.
From www.numerade.com
SOLVED Compare the compressibility of solids and liquids. Support your Solid Liquid Compressibility Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how liquids can be compressed by pressure and temperature,. Solid Liquid Compressibility.
From slideplayer.com
Liquids and Solids Gas Solid low density high compressibility ppt Solid Liquid Compressibility Learn how the kinetic molecular theory of gases can be applied to liquids, taking into account the nonzero volumes and strong intermolecular forces of liquids. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn how. Solid Liquid Compressibility.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Solid Liquid Compressibility Explore the factors that affect the. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how the kinetic. Solid Liquid Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid Compressibility Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Explore the factors that affect the. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Learn how particles are. Solid Liquid Compressibility.
From slideplayer.com
Unit 7 States of matter and the Behavior of Gases ppt download Solid Liquid Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Learn the definition and examples of solids, liquids and gases, and how they differ in compressibility. Learn about the compressibility of solids, liquids and gases, and how it relates to pressure and volume. Gases are highly compressible compared to solids and liquids due. Solid Liquid Compressibility.
From brainly.in
State the reason behind the observation recorded in the activity to Solid Liquid Compressibility Learn how to define and calculate the isothermal compressibility and isobaric thermal expansivity of solids, liquids and. Gases are highly compressible compared to solids and liquids due to the larger. Learn how particles are arranged and move in solids, liquids and gases using the particle model of matter. Learn how liquids can be compressed by pressure and temperature, and how. Solid Liquid Compressibility.