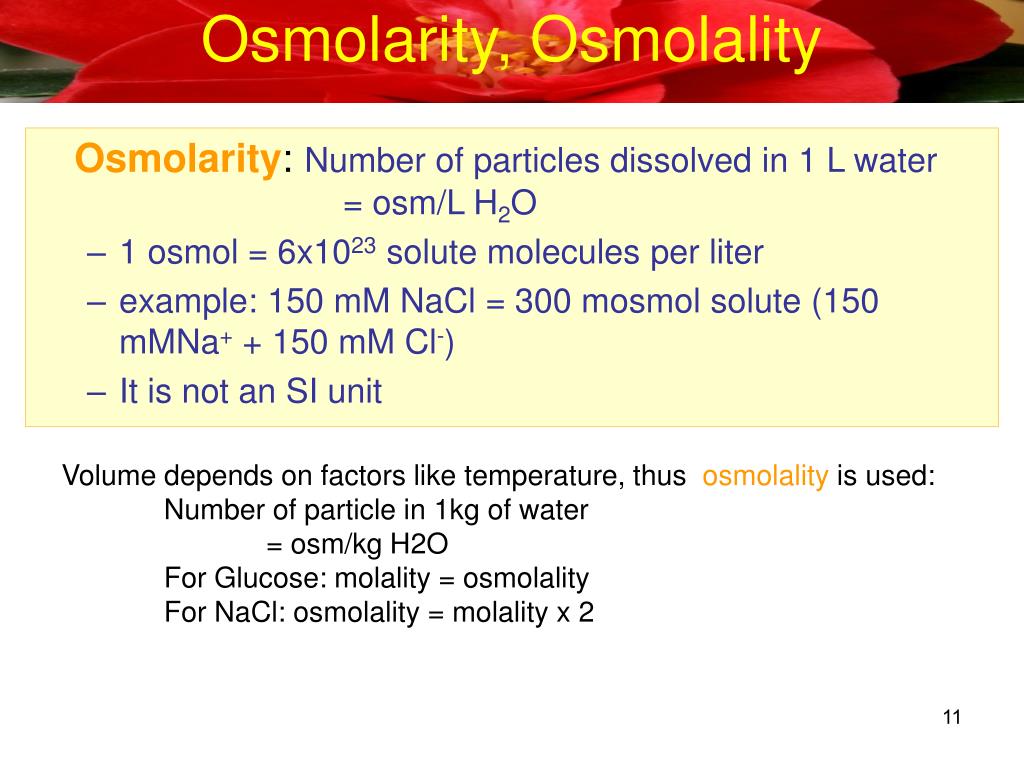
What Is The Osmolarity Of Distilled Water . This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. You multiply the molarity by the number of osmoles that each solute produces. How do you calculate osmolarity of a solution? An osmole is 1 mol of particles that contribute to the osmotic pressure of a. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Learn what osmolarity and osmolality are and how to express them. Osmolarity is the number of osmoles of solute per litre of solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids.
from www.slideserve.com
Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate osmolarity of a solution? You multiply the molarity by the number of osmoles that each solute produces. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An osmole is 1 mol of particles that contribute to the osmotic pressure of a.
PPT Water Metabolism PowerPoint Presentation, free download ID4493121
What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. How do you calculate osmolarity of a solution? Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Osmolarity is the number of osmoles of solute per litre of solution.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Osmolarity and osmolality are. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT BODY FLUID COMPARTMENT AND FLUID BALANCE PowerPoint Presentation What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity is the number of osmoles of solute per litre of solution. While any polar solvent could be used, these. What Is The Osmolarity Of Distilled Water.
From www.slideshare.net
Formula osmolality and nutritional needs What Is The Osmolarity Of Distilled Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Learn what osmolarity and osmolality are and how to express them. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1. What Is The Osmolarity Of Distilled Water.
From quizlet.com
changes in osmolarity of tubular fluid Diagram Quizlet What Is The Osmolarity Of Distilled Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. An osmole is 1 mol of particles that contribute to the osmotic pressure of. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
BODY FLUID VOLUME OSMOLARITY DIAGRAM YouTube What Is The Osmolarity Of Distilled Water While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. You multiply the molarity by the number of osmoles that each solute produces. This means a. What Is The Osmolarity Of Distilled Water.
From blog.healthmatters.io
What is Osmolality? HealthMatters.io Lab results explained What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Osmolarity is the number of osmoles of solute per. What Is The Osmolarity Of Distilled Water.
From www.chegg.com
Solved Table III Osmolarity Summary Table Isotonic What Is The Osmolarity Of Distilled Water You multiply the molarity by the number of osmoles that each solute produces. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units. What Is The Osmolarity Of Distilled Water.
From whatiswaterwebsite.com
What Is The Osmolarity Of Distilled Water What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Learn what osmolarity and osmolality are and how to express them. This means a solution with 1 mol/l nacl has an osmolarity. What Is The Osmolarity Of Distilled Water.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation What Is The Osmolarity Of Distilled Water While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity is the number of osmoles of solute per litre of solution. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Is The Osmolarity Of Distilled Water This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. How do you calculate osmolarity of a solution? While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply. What Is The Osmolarity Of Distilled Water.
From droualb.faculty.mjc.edu
Chapter 4 What Is The Osmolarity Of Distilled Water While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. How do you calculate osmolarity of a solution? Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute. What Is The Osmolarity Of Distilled Water.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary What Is The Osmolarity Of Distilled Water Osmolarity is the number of osmoles of solute per litre of solution. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. How do you calculate osmolarity of a solution? Learn what osmolarity and osmolality are and how. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry What Is The Osmolarity Of Distilled Water Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate osmolarity of a solution? While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Learn what osmolarity and. What Is The Osmolarity Of Distilled Water.
From www.chegg.com
Solved 8. What is the osmolarity for 1 1 liter of solution What Is The Osmolarity Of Distilled Water You multiply the molarity by the number of osmoles that each solute produces. Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate osmolarity of a solution? While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. This means a solution with 1 mol/l nacl has an. What Is The Osmolarity Of Distilled Water.
From slidetodoc.com
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration What Is The Osmolarity Of Distilled Water You multiply the molarity by the number of osmoles that each solute produces. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
Osmolarity Example (Bio) YouTube What Is The Osmolarity Of Distilled Water This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Osmoregulation = keeping water and salt balanced in the body What Is The Osmolarity Of Distilled Water Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Membrane Dynamics Movement and Potential PowerPoint Presentation What Is The Osmolarity Of Distilled Water This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Learn what. What Is The Osmolarity Of Distilled Water.
From www.researchgate.net
Composition and osmolarity (Osm/L) of DSW and NaCl solutions. Figures What Is The Osmolarity Of Distilled Water This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. How do you calculate osmolarity of a solution? Osmolarity is the number of osmoles of solute per litre of solution.. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. How do. What Is The Osmolarity Of Distilled Water.
From drawittoknowit.com
Cell Biology Osmosis and Osmolarity ditki medical & biological sciences What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these units are used almost exclusively for aqueous. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. How do you calculate osmolarity of a solution? You multiply the molarity by the number of osmoles that each solute produces. Osmolarity and osmolality are units of solute concentration that are often. What Is The Osmolarity Of Distilled Water.
From quenchwater.com
Distilled Water vs Purified Water Quench Water What Is The Osmolarity Of Distilled Water Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate osmolarity of a solution? This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used,. What Is The Osmolarity Of Distilled Water.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical What Is The Osmolarity Of Distilled Water While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. How do you calculate osmolarity of a solution? This means a. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Learn what osmolarity and osmolality are and how to express them. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole is 1 mol of particles that. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
How to solve osmolarity calculation problems YouTube What Is The Osmolarity Of Distilled Water You multiply the molarity by the number of osmoles that each solute produces. Learn what osmolarity and osmolality are and how to express them. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. How do you. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT Osmoregulation PowerPoint Presentation, free download ID2119652 What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. You multiply the. What Is The Osmolarity Of Distilled Water.
From www.goconqr.com
Regulation of Water Balance ( Osmolarity ) Mind Map What Is The Osmolarity Of Distilled Water While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. How do you calculate osmolarity of a solution? Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol. What Is The Osmolarity Of Distilled Water.
From www.slideshare.net
7Membrane Transport I What Is The Osmolarity Of Distilled Water You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Learn what osmolarity and osmolality are and how to express them. An osmole is 1 mol. What Is The Osmolarity Of Distilled Water.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki What Is The Osmolarity Of Distilled Water This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Learn what osmolarity and osmolality are and how to express them. Osmolarity is the number of osmoles of solute per litre of solution. While any polar solvent could be used, these. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube What Is The Osmolarity Of Distilled Water Learn what osmolarity and osmolality are and how to express them. Osmolarity is the number of osmoles of solute per litre of solution. How do you calculate osmolarity of a solution? You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. This. What Is The Osmolarity Of Distilled Water.
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 What Is The Osmolarity Of Distilled Water How do you calculate osmolarity of a solution? Osmolarity is the number of osmoles of solute per litre of solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that. What Is The Osmolarity Of Distilled Water.
From slideshare.net
Diffusion Osmosis What Is The Osmolarity Of Distilled Water An osmole is 1 mol of particles that contribute to the osmotic pressure of a. Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. How do you calculate osmolarity of a solution? You multiply the molarity by the number of osmoles. What Is The Osmolarity Of Distilled Water.
From www.youtube.com
WCLN Osmosis water sugar solution Biology YouTube What Is The Osmolarity Of Distilled Water An osmole is 1 mol of particles that contribute to the osmotic pressure of a. How do you calculate osmolarity of a solution? This means a solution with 1 mol/l nacl has an osmolarity of 2 osmol/l. Osmolarity is the number of osmoles of solute per litre of solution. Learn what osmolarity and osmolality are and how to express them.. What Is The Osmolarity Of Distilled Water.
From www.chegg.com
Solved (Distilled water, 150 or 1000 mM NaCT) Osmolarity of What Is The Osmolarity Of Distilled Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An osmole is 1 mol of particles that contribute to the osmotic pressure of a. You multiply the molarity by the number of osmoles. What Is The Osmolarity Of Distilled Water.