What Reacts With Sulfur . Sulfur compounds have many unusual properties, including the ability to catenate like carbon. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. the reaction between magnesium and sulfur is typical. S 8 (s) + 12 koh (aq) 4 k 2. — sulfur, nonmetallic chemical element, one of the most reactive of the elements. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). It reacts with all metals except gold and platinum, forming sulfides. S 8 (s) + 6koh(aq). sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. When the two elements are heated, they combine to form magnesium sulfide (mgs):
from www.transtutors.com
— except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. S 8 (s) + 6koh(aq). It reacts with all metals except gold and platinum, forming sulfides. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. When the two elements are heated, they combine to form magnesium sulfide (mgs): — sulfur, nonmetallic chemical element, one of the most reactive of the elements. the reaction between magnesium and sulfur is typical. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−.
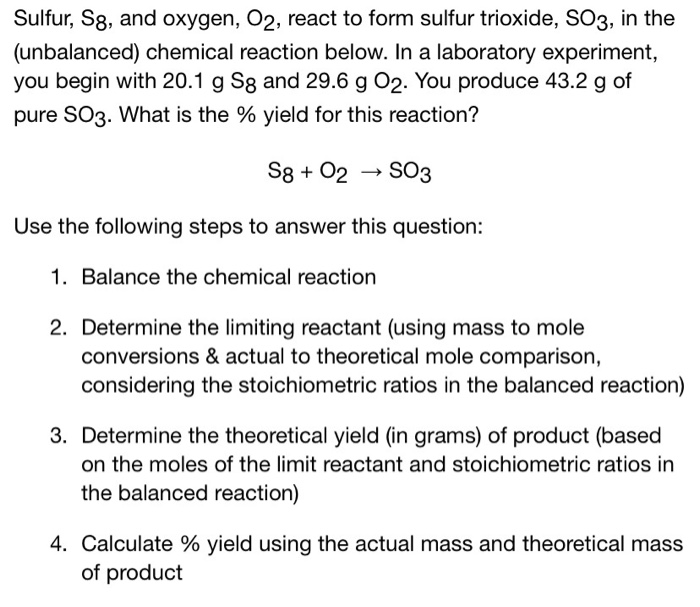
(Solved) Sulfur, S8, And Oxygen, O2, React To Form Sulfur Trioxide
What Reacts With Sulfur the reaction between magnesium and sulfur is typical. the reaction between magnesium and sulfur is typical. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. It reacts with all metals except gold and platinum, forming sulfides. — sulfur, nonmetallic chemical element, one of the most reactive of the elements. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. S 8 (s) + 12 koh (aq) 4 k 2. When the two elements are heated, they combine to form magnesium sulfide (mgs): S 8 (s) + 6koh(aq). sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species.
From www.sciencephoto.com
Iron reacting with sulphur Stock Image A500/0375 Science Photo Library What Reacts With Sulfur Sulfur compounds have many unusual properties, including the ability to catenate like carbon. S 8 (s) + 6koh(aq). When the two elements are heated, they combine to form magnesium sulfide (mgs): — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). sulphur reacts with hot aqueous potassium hydroxide,. What Reacts With Sulfur.
From www.slideserve.com
PPT Sulfur Dioxide PowerPoint Presentation, free download ID6885076 What Reacts With Sulfur the reaction between magnesium and sulfur is typical. It reacts with all metals except gold and platinum, forming sulfides. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including. What Reacts With Sulfur.
From www.youtube.com
Oleum. Sulfur trioxide SO3. Chemical reactions YouTube What Reacts With Sulfur sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. When the two elements are heated, they combine to form magnesium sulfide (mgs): the reaction between magnesium and sulfur is typical. . What Reacts With Sulfur.
From www.numerade.com
SOLVEDSulfur dioxide gas reacts with oxygen, O2(g), to produce SO3(g What Reacts With Sulfur the reaction between magnesium and sulfur is typical. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). It reacts with all metals except gold and platinum, forming sulfides. S 8 (s) + 12 koh. What Reacts With Sulfur.
From www.vrogue.co
Solved Chemwork Sulfur Dioxide Gas Reacts With Sodium vrogue.co What Reacts With Sulfur sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: the reaction between magnesium and sulfur is typical. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. It reacts with all metals except gold and platinum, forming sulfides. — except for noble gases, sulfur is highly reactive and. What Reacts With Sulfur.
From www.chegg.com
Sulfur Dioxide Will React With Water To Form Sulfu... What Reacts With Sulfur sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). S 8 (s) + 12 koh (aq) 4 k 2. . What Reacts With Sulfur.
From aidankruwmontes.blogspot.com
The Reaction of 1 Mole of Sulfur Dioxide With Oxygen AidankruwMontes What Reacts With Sulfur — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: It reacts with all metals except gold and platinum, forming sulfides. S 8 (s) + 12 koh (aq) 4 k 2. S 8 (s) +. What Reacts With Sulfur.
From www.numerade.com
SOLVED(a) Sulfur, S8, combines with oxygen at elevated temper atures What Reacts With Sulfur S 8 (s) + 6koh(aq). S 8 (s) + 12 koh (aq) 4 k 2. the reaction between magnesium and sulfur is typical. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. sulphur reacts with hot aqueous potassium hydroxide, koh, to. What Reacts With Sulfur.
From www.vrogue.co
Solved Chemwork Sulfur Dioxide Gas Reacts With Sodium vrogue.co What Reacts With Sulfur S 8 (s) + 6koh(aq). sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. It reacts with all metals except gold and platinum, forming sulfides. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. sulfur (group 16) reacts with. What Reacts With Sulfur.
From www.sciencephoto.com
Iron reacts with sulphur Stock Image C036/3054 Science Photo Library What Reacts With Sulfur the reaction between magnesium and sulfur is typical. When the two elements are heated, they combine to form magnesium sulfide (mgs): S 8 (s) + 6koh(aq). It reacts with all metals except gold and platinum, forming sulfides. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: sulphur reacts with hot aqueous potassium hydroxide,. What Reacts With Sulfur.
From www.slideserve.com
PPT LECTURE 9 SULFUR AND SULFURIC ACID PowerPoint Presentation, free What Reacts With Sulfur the reaction between magnesium and sulfur is typical. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. S 8 (s) + 6koh(aq). sulphur. What Reacts With Sulfur.
From www.coursehero.com
[Solved] Iron (Fe) reacts with sulfur (S8) to produce iron(II) sulfide What Reacts With Sulfur S 8 (s) + 6koh(aq). When the two elements are heated, they combine to form magnesium sulfide (mgs): S 8 (s) + 12 koh (aq) 4 k 2. the reaction between magnesium and sulfur is typical. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: sulphur reacts with hot aqueous potassium hydroxide, koh,. What Reacts With Sulfur.
From www.slideshare.net
Unit 2 Classifying Matter What Reacts With Sulfur — sulfur, nonmetallic chemical element, one of the most reactive of the elements. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. — except for noble. What Reacts With Sulfur.
From www.youtube.com
Reaction between sodium and sulfur YouTube What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. S 8 (s) + 6koh(aq). When the two. What Reacts With Sulfur.
From www.transtutors.com
(Solved) Sulfur, S8, And Oxygen, O2, React To Form Sulfur Trioxide What Reacts With Sulfur sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. — sulfur, nonmetallic chemical element, one of the most reactive of the elements. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. Sulfur compounds have many unusual. What Reacts With Sulfur.
From study.com
Sulfur Trioxide Formula, Reactions & Preparation Lesson What Reacts With Sulfur sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: — sulfur, nonmetallic chemical. What Reacts With Sulfur.
From www.britannica.com
sulfur Definition, Element, Symbol, Uses, & Facts Britannica What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: Sulfur compounds have many unusual properties, including the ability to catenate like carbon. . What Reacts With Sulfur.
From chemistryiitjamnetset.blogspot.com
IIT JAM UGC CSIR NET GATE CHEMISTRY Reaction of sugar with sulfuric acid What Reacts With Sulfur When the two elements are heated, they combine to form magnesium sulfide (mgs): S 8 (s) + 12 koh (aq) 4 k 2. the reaction between magnesium and sulfur is typical. S 8 (s) + 6koh(aq). It reacts with all metals except gold and platinum, forming sulfides. sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide. What Reacts With Sulfur.
From www.gaylordchemical.com
Sulfur Ylide Reactions Gaylord Chemical What Reacts With Sulfur S 8 (s) + 6koh(aq). Sulfur compounds have many unusual properties, including the ability to catenate like carbon. the reaction between magnesium and sulfur is typical. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. — except for noble gases, sulfur is highly reactive. What Reacts With Sulfur.
From www.slideserve.com
PPT Sulfur oxides PowerPoint Presentation, free download ID4172151 What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. the reaction between magnesium and sulfur is typical. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. When the two elements. What Reacts With Sulfur.
From www.youtube.com
REACTION OF IRON WITH SULPHUR YouTube What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. When the two elements are heated, they combine to form magnesium sulfide (mgs): the reaction between magnesium and sulfur is typical. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color,. What Reacts With Sulfur.
From www.youtube.com
Copper and Sulfur reaction YouTube What Reacts With Sulfur S 8 (s) + 12 koh (aq) 4 k 2. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. When the two elements are heated, they combine to form magnesium sulfide (mgs): — sulfur, nonmetallic chemical element, one of the most reactive. What Reacts With Sulfur.
From thechemistrynotes.com
Sulfur (S) Element History, Properties, Uses, Reactions, Safety What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. When the two elements are heated, they combine to form magnesium sulfide (mgs): — sulfur, nonmetallic chemical element, one of the most reactive of the elements. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as. What Reacts With Sulfur.
From www.sarthaks.com
Sulphur reacts with chlorine in `12` ratio and forms X hydrolysis of X What Reacts With Sulfur When the two elements are heated, they combine to form magnesium sulfide (mgs): S 8 (s) + 12 koh (aq) 4 k 2. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). the reaction between magnesium and sulfur is typical. It reacts with all metals except gold. What Reacts With Sulfur.
From www.chegg.com
Solved pFluoroanisole reacts with sulfur trioxide and What Reacts With Sulfur sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. — except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal). — sulfur, nonmetallic chemical element, one of the most reactive of the elements. S 8 (s) + 12 koh (aq) 4 k 2.. What Reacts With Sulfur.
From kunduz.com
[ANSWERED] What reacts with sulfur dioxide in the atmosphere to form What Reacts With Sulfur S 8 (s) + 6koh(aq). sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. — sulfur, nonmetallic chemical element, one of the most reactive of the elements. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: . What Reacts With Sulfur.
From www.nagwa.com
Question Video Reaction of Sulfur to Generate a Feedstock Gas for the What Reacts With Sulfur S 8 (s) + 12 koh (aq) 4 k 2. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. When the two elements are heated, they combine to form magnesium sulfide (mgs): — except for noble gases, sulfur is highly reactive and. What Reacts With Sulfur.
From www.studocu.com
Reactions of liquid Sulfur dioxide Sulfur dioxide (SO2) Definition What Reacts With Sulfur sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. sulfur reacts with hot aqueous potassium hydroxide, koh, forming potassium sulfide and thiosulfate: Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. When the two elements are heated, they combine. What Reacts With Sulfur.
From www.sciencephoto.com
Iron and sulfur reaction Stock Image C029/1071 Science Photo Library What Reacts With Sulfur the reaction between magnesium and sulfur is typical. S 8 (s) + 6koh(aq). When the two elements are heated, they combine to form magnesium sulfide (mgs): sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in. What Reacts With Sulfur.
From www.sciencephoto.com
Iron reacts with sulphur, 2 of 4 Stock Image C036/3056 Science What Reacts With Sulfur S 8 (s) + 12 koh (aq) 4 k 2. S 8 (s) + 6koh(aq). Sulfur compounds have many unusual properties, including the ability to catenate like carbon. It reacts with all metals except gold and platinum, forming sulfides. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble. What Reacts With Sulfur.
From www.slideserve.com
PPT Sulfur Cycle PowerPoint Presentation, free download ID2501965 What Reacts With Sulfur S 8 (s) + 6koh(aq). sulphur reacts with hot aqueous potassium hydroxide, koh, to form sulphide and thiosulphate species. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. Pure. What Reacts With Sulfur.
From www.youtube.com
Sodium and Sulfur Reaction YouTube What Reacts With Sulfur Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. the reaction between magnesium and sulfur is typical. Sulfur compounds have many unusual properties, including the ability to catenate like carbon. S 8 (s) + 6koh(aq). When the two elements are heated, they combine to form. What Reacts With Sulfur.
From www.numerade.com
Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite What Reacts With Sulfur It reacts with all metals except gold and platinum, forming sulfides. sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. S 8 (s) + 12 koh (aq) 4 k 2. — except for noble gases, sulfur is highly reactive and almost reacts. What Reacts With Sulfur.
From solvedlib.com
The reaction of Copper (V) sulfide reacts with sulfur… SolvedLib What Reacts With Sulfur — sulfur, nonmetallic chemical element, one of the most reactive of the elements. S 8 (s) + 6koh(aq). sulfur (group 16) reacts with almost all metals and readily forms the sulfide ion, s 2−, in which it has as oxidation state of 2−. — except for noble gases, sulfur is highly reactive and almost reacts with all. What Reacts With Sulfur.
From www.youtube.com
Redox Reaction Iodine with Sulphur Dioxide in Acidic Medium YouTube What Reacts With Sulfur When the two elements are heated, they combine to form magnesium sulfide (mgs): Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a poor conductor of electricity, and insoluble in water. It reacts with all metals except gold and platinum, forming sulfides. — except for noble gases, sulfur is highly reactive and almost reacts. What Reacts With Sulfur.