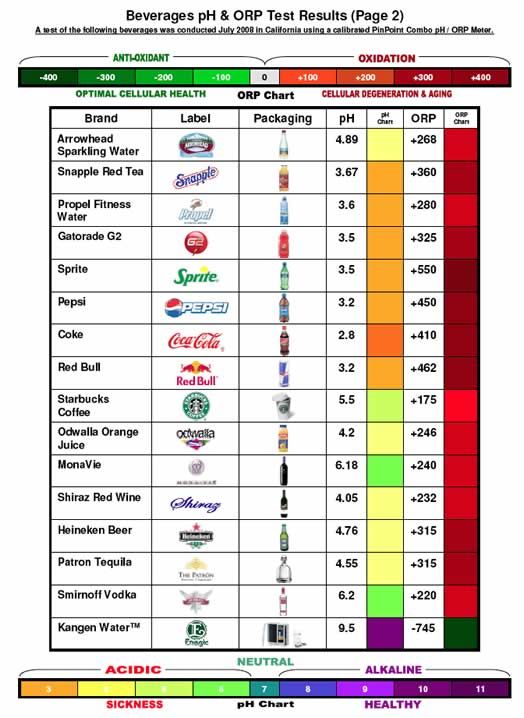
Is Carbonated Water Acid Or Base . Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the. The ph of the water you drink does not affect the ph of your blood. Equation \ (\pageindex {1}\) ), and a base. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Use this acids and bases chart to find the relative strength of the most common acids and bases. Is carbonated water is it acid or base? Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction.
from www.pinterest.com
Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Is carbonated water is it acid or base? The ph of the water you drink does not affect the ph of your blood. Use this acids and bases chart to find the relative strength of the most common acids and bases. Equation \ (\pageindex {1}\) ), and a base. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the.
pH of soft drinks Alkaline diet, Alkaline foods, Diet
Is Carbonated Water Acid Or Base The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Is carbonated water is it acid or base? The ph of the water you drink does not affect the ph of your blood. Equation \ (\pageindex {1}\) ), and a base. Use this acids and bases chart to find the relative strength of the most common acids and bases. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water.
From gbu-presnenskij.ru
Sparkling Water Benefits What Is Sparkling Water? Is It, 59 OFF Is Carbonated Water Acid Or Base Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Use this acids and bases chart to find the relative strength of the most common acids and bases. Equation \ (\pageindex {1}\). Is Carbonated Water Acid Or Base.
From crosswordlabs.com
Acids, Bases and Salts Crossword Labs Is Carbonated Water Acid Or Base Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Equation \ (\pageindex {1}\) ), and a base.. Is Carbonated Water Acid Or Base.
From crosswordlabs.com
Acids Bases and Salts Crossword Labs Is Carbonated Water Acid Or Base Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Use this acids and bases chart to find the relative strength of the most common acids and bases. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Is carbonated water is it acid or base? According to the arrhenius definition,. Is Carbonated Water Acid Or Base.
From sciencenotes.org
The pH Scale of Common Chemicals Is Carbonated Water Acid Or Base Use this acids and bases chart to find the relative strength of the most common acids and bases. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Equation \ (\pageindex {1}\) ), and a base. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Is carbonated water is it. Is Carbonated Water Acid Or Base.
From giobngmpe.blob.core.windows.net
What Happens When Base Reacts With Carbon Dioxide at Audrey Lovejoy blog Is Carbonated Water Acid Or Base Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Water is not the only substance that can react as an acid in some cases or. Is Carbonated Water Acid Or Base.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Is Carbonated Water Acid Or Base Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the. Is carbonated water is it acid or base? Use this acids and bases chart to find the relative strength of the most common acids and bases. The h 2 co 3. Is Carbonated Water Acid Or Base.
From giobngmpe.blob.core.windows.net
What Happens When Base Reacts With Carbon Dioxide at Audrey Lovejoy blog Is Carbonated Water Acid Or Base The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. The ph of the water you drink does not affect the ph of your blood. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. Water is not the only substance that. Is Carbonated Water Acid Or Base.
From kidspressmagazine.com
Acids and Bases Is Carbonated Water Acid Or Base According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. The h 2 co 3 is a product. Is Carbonated Water Acid Or Base.
From www.priyamstudycentre.com
Sodium Carbonate NaCO3 Compound, Uses Is Carbonated Water Acid Or Base The ph of the water you drink does not affect the ph of your blood. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the. Equation. Is Carbonated Water Acid Or Base.
From www.dreamstime.com
Carbonic Acid H2CO3 Molecule . it is Also Solution of Carbon Stock Is Carbonated Water Acid Or Base Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. The ph of the water you drink does not affect the ph of your blood. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Is carbonated water is it acid or. Is Carbonated Water Acid Or Base.
From www.dreamstime.com
Carbonic Acid Molecule. Formed when Carbon Dioxide is Dissolved in Is Carbonated Water Acid Or Base Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Equation \ (\pageindex {1}\) ), and a base. According to the arrhenius definition, an acid is a substance like hydrochloric acid. Is Carbonated Water Acid Or Base.
From www.newhealthadvisor.org
Is Carbonation Bad for You? New Health Advisor Is Carbonated Water Acid Or Base According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Water is not the only substance that can react as an acid in some cases or a base in others, but it. Is Carbonated Water Acid Or Base.
From tastylicious.com
Is Carbonated (Sparkling) Water Acidic? Tastylicious Is Carbonated Water Acid Or Base Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Is carbonated water is it acid or base? Sparkling water is slightly acidic because it’s infused with carbon dioxide to make. Is Carbonated Water Acid Or Base.
From thechemicalfreewoods.blogspot.com
The ChemicalFree Woods Is Carbonated Water Acid Or Base The ph of the water you drink does not affect the ph of your blood. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Water is not the only substance that can react as an acid in some cases or a base in others, but. Is Carbonated Water Acid Or Base.
From giobngmpe.blob.core.windows.net
What Happens When Base Reacts With Carbon Dioxide at Audrey Lovejoy blog Is Carbonated Water Acid Or Base Equation \ (\pageindex {1}\) ), and a base. According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Is carbonated water is it acid or base? Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Water is not the only substance. Is Carbonated Water Acid Or Base.
From www.pinterest.com
pH of soft drinks Alkaline diet, Alkaline foods, Diet Is Carbonated Water Acid Or Base According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Is carbonated water is it acid or base? The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. The ph of the water. Is Carbonated Water Acid Or Base.
From lessondbunsublimed.z21.web.core.windows.net
Type Of Acid Base Neutralization Reactions Is Carbonated Water Acid Or Base Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Use this acids and bases chart to find the relative strength of the most common acids and bases. The ph of the water you drink does not affect the ph of your blood. Equation \ (\pageindex {1}\) ), and a base. Is carbonated water is. Is Carbonated Water Acid Or Base.
From appliednutrition.uk
Protein Sparkling Water Can (330ml) Applied Nutrition Ltd Is Carbonated Water Acid Or Base According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Use this acids and bases chart to find the relative strength of the most common acids and bases. The ph of the water you drink does not affect the ph of your blood. The h 2 co. Is Carbonated Water Acid Or Base.
From www.cbc.ca
Marketplace tested Perrier, LaCroix, Bubly sparkling waters to see Is Carbonated Water Acid Or Base Carbonic acid (h 2 co 3) is a weak acid found in carbonated water. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Use this acids and bases chart to. Is Carbonated Water Acid Or Base.
From tastylicious.com
Is Carbonated Water Acidic? Tastylicious Is Carbonated Water Acid Or Base Is carbonated water is it acid or base? Use this acids and bases chart to find the relative strength of the most common acids and bases. The h 2 co 3 is a product of carbon dioxide (co 2 ) and water (h 2 o) by the following equilibrium reaction. Carbonated water is, in simple terms, water that has had. Is Carbonated Water Acid Or Base.
From in.pinterest.com
pH chart Skins ph, Acid base, Carbonated drinks Is Carbonated Water Acid Or Base Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Use this acids and bases chart to find the relative strength of the most common acids and bases. The h 2 co 3 is a product of carbon dioxide (co. Is Carbonated Water Acid Or Base.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Is Carbonated Water Acid Or Base Use this acids and bases chart to find the relative strength of the most common acids and bases. Carbonated water is, in simple terms, water that has had carbon dioxide forced into it. Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. The h 2 co 3 is a product of carbon dioxide (co. Is Carbonated Water Acid Or Base.
From isabellegordon.z19.web.core.windows.net
Bottled Water Ph Level Chart Is Carbonated Water Acid Or Base According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Use this acids and bases chart to find the relative strength of the most common acids and bases. Is carbonated water is. Is Carbonated Water Acid Or Base.
From www.lorca.vn
Tác hại của nước có gas đối với sức khỏe Bếp từ Lorca an toàn tối đa Is Carbonated Water Acid Or Base Sparkling water is slightly acidic because it’s infused with carbon dioxide to make it bubbly. Is carbonated water is it acid or base? According to the arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce h + ions (protons; The h 2 co 3 is a product of carbon dioxide (co 2 ). Is Carbonated Water Acid Or Base.