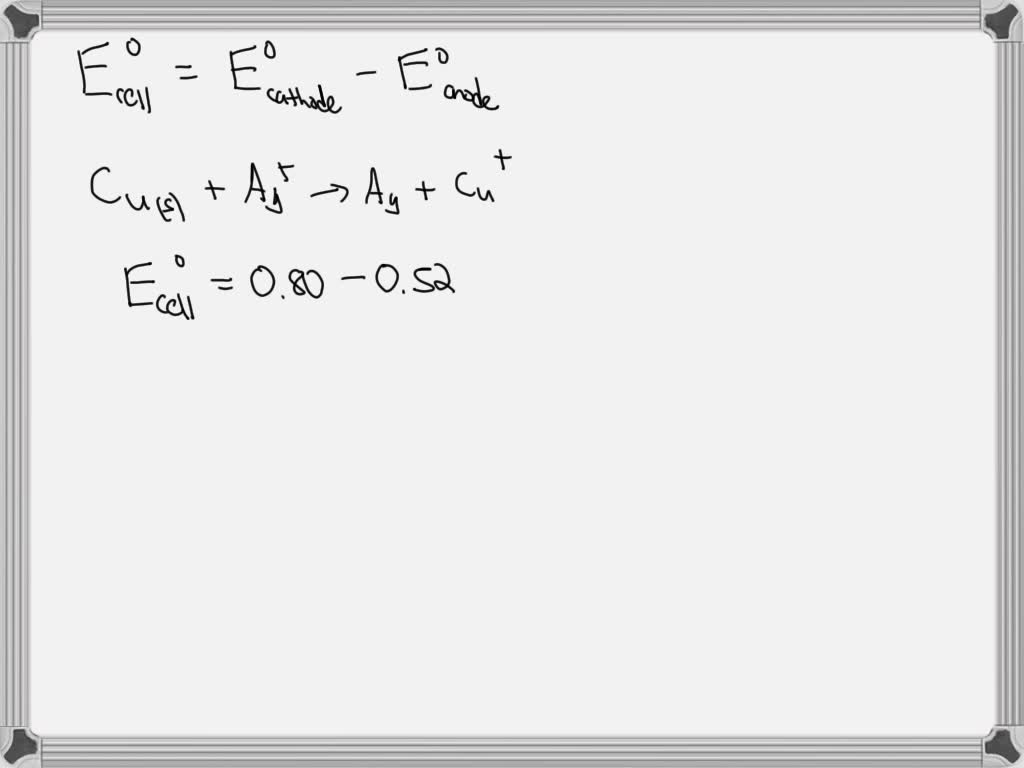Standard Cell Potential Conditions . Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. How to use a table of standard reduction potentials to calculate standard cell potential. The standard cell potential is a measure of the driving force for the reaction.
from www.numerade.com
The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is a measure of the driving force for the reaction. How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Identifying trends in oxidizing and reducing agent strength.
SOLVED 1 Calculate the standard cell potential (from the Standard
Standard Cell Potential Conditions How to use a table of standard reduction potentials to calculate standard cell potential. How to use a table of standard reduction potentials to calculate standard cell potential. The standard cell potential is a measure of the driving force for the reaction. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Identifying. Standard Cell Potential Conditions.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Conditions Since the definition of cell. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in. Standard Cell Potential Conditions.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Since the definition of cell. The standard cell potential is a measure of the driving force for the reaction. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Cell Potential Conditions Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is. Standard Cell Potential Conditions.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Cell Potential Conditions Since the definition of cell. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is a measure of the driving force for the reaction. How to use a table of standard. Standard Cell Potential Conditions.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. How to use a table of standard reduction potentials to calculate standard cell potential.. Standard Cell Potential Conditions.
From slideplayer.com
Chapter 20 Electrochemistry ppt download Standard Cell Potential Conditions How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is a measure of the driving force for the reaction. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to measure and interpret. Standard Cell Potential Conditions.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is a measure of the driving force for the reaction. Learn how to measure and interpret electrode. Standard Cell Potential Conditions.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. How to use a table of standard reduction potentials to calculate standard cell potential. The standard cell potential is a measure of the driving force for the reaction. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the. Standard Cell Potential Conditions.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Conditions How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Conditions.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Identifying trends in oxidizing and reducing agent strength. The standard. Standard Cell Potential Conditions.
From www.chegg.com
Solved For each reaction listed, determine its standard cell Standard Cell Potential Conditions Since the definition of cell. The standard cell potential is a measure of the driving force for the reaction. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Identifying trends in oxidizing and reducing agent strength. How to use a table of standard reduction potentials. Standard Cell Potential Conditions.
From pveducation.org
Standard Potential PVEducation Standard Cell Potential Conditions Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances). Standard Cell Potential Conditions.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard. Standard Cell Potential Conditions.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. Learn how to measure and interpret electrode and cell potentials, which reflect. Standard Cell Potential Conditions.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. The standard cell potential is a measure of the driving force for the reaction. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode and cell potentials, which reflect. Standard Cell Potential Conditions.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Identifying trends in oxidizing and reducing agent strength. Learn how to measure and interpret electrode. Standard Cell Potential Conditions.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Standard Cell Potential Conditions Since the definition of cell. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1. Standard Cell Potential Conditions.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. The standard cell potential is a measure of the driving force for the reaction. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. How to use a table of standard. Standard Cell Potential Conditions.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. Since the definition of cell. How to use a table of standard reduction potentials to calculate standard cell potential. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Since the definition of cell. How to use a table of standard reduction potentials to calculate standard cell potential. Identifying trends in oxidizing and reducing agent strength. Learn how to measure and interpret electrode. Standard Cell Potential Conditions.
From www.chegg.com
Solved For each reaction listed, determine its standard cell Standard Cell Potential Conditions Since the definition of cell. Identifying trends in oxidizing and reducing agent strength. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the driving force for the reaction. The potential of the cell under standard conditions (1. Standard Cell Potential Conditions.
From joipgxufc.blob.core.windows.net
Standard Cell Potential Vs Cell Potential at Matthew Bryant blog Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1. Standard Cell Potential Conditions.
From www.researchgate.net
Values of the standard potentials of cerium redox systems in anodic Standard Cell Potential Conditions Identifying trends in oxidizing and reducing agent strength. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. How to. Standard Cell Potential Conditions.
From www.youtube.com
Electrochemistry 05 Calculating Standard Cell Potentials YouTube Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. The standard cell potential is a measure of the driving. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Since the definition of cell. The standard cell potential is a measure of the driving force for the reaction. How to use a table of standard reduction potentials to calculate standard cell potential. The potential of. Standard Cell Potential Conditions.
From www.youtube.com
How to find the cell potential (Ecell) under standard conditions YouTube Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Learn how to measure and interpret electrode. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. How to use a table of standard reduction potentials to calculate standard. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. The standard cell potential is. Standard Cell Potential Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Cell Potential Conditions The standard cell potential is a measure of the driving force for the reaction. Since the definition of cell. How to use a table of standard reduction potentials to calculate standard cell potential. Identifying trends in oxidizing and reducing agent strength. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Conditions.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Conditions Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Identifying trends in oxidizing and reducing agent strength. How to use a table of standard reduction potentials to calculate standard cell potential. The standard cell potential is a measure. Standard Cell Potential Conditions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Conditions The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Identifying trends in oxidizing and reducing agent strength. How to use a table of standard reduction potentials to calculate standard cell potential. Since the definition of cell. The standard cell potential is a measure. Standard Cell Potential Conditions.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Standard Cell Potential Conditions Learn how to measure and interpret electrode and cell potentials, which reflect the redox activity of species and the driving force for electron transfer. How to use a table of standard reduction potentials to calculate standard cell potential. The standard cell potential is a measure of the driving force for the reaction. Identifying trends in oxidizing and reducing agent strength.. Standard Cell Potential Conditions.