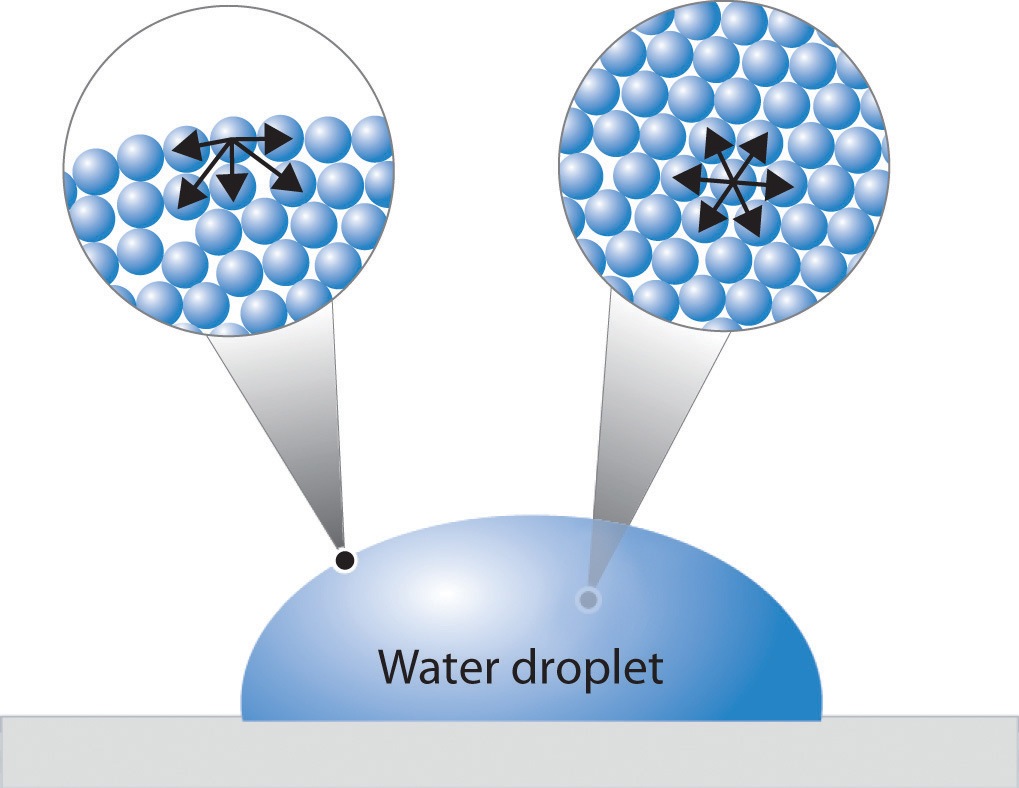
High Surface Tension Polar . The polarity of water molecules can help explain why. The attraction of molecules at the surface of a liquid is called surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension is the energy required to increase the surface area of a liquid by a given amount. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The stronger the intermolecular interactions, the greater the surface tension. The surface tension of water. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. the surface tension of water is 72 dynes/cm at 25°c.
from chem.libretexts.org
The polarity of water molecules can help explain why. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The attraction of molecules at the surface of a liquid is called surface tension. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. The stronger the intermolecular interactions, the greater the surface tension. the surface tension of water is 72 dynes/cm at 25°c. The surface tension of water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension is the energy required to increase the surface area of a liquid by a given amount.
Surface Tension Chemistry LibreTexts
High Surface Tension Polar The surface tension of water. The attraction of molecules at the surface of a liquid is called surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why. The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy required to increase the surface area of a liquid by a given amount. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The surface tension of water. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. the surface tension of water is 72 dynes/cm at 25°c.
From www.researchgate.net
Twodimensional polar plots of surface tension on different planes (a)... Download Scientific High Surface Tension Polar the surface tension of water is 72 dynes/cm at 25°c. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The surface tension of water. It. High Surface Tension Polar.
From www.pnas.org
Turning traditionally nonwetting surfaces wetting for even ultrahigh surface energy liquids PNAS High Surface Tension Polar The surface tension of water. The polarity of water molecules can help explain why. the surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The attraction of molecules at the surface of a liquid is called surface tension. The stronger the. High Surface Tension Polar.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of water is 72 dynes/cm at 25°c. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water.. High Surface Tension Polar.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts High Surface Tension Polar surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is the energy, or work, required to increase. High Surface Tension Polar.
From www.researchgate.net
First row polar plot of surface tension, i.e. the function σ ̄ (θ )... Download Scientific High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water. The polarity of water molecules can help explain why. surface tension, heat. High Surface Tension Polar.
From www.slideserve.com
PPT Limnology! PowerPoint Presentation, free download ID1426315 High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of water is 72 dynes/cm at 25°c. The attraction of molecules at the surface of a liquid is called surface tension. surface tension, heat. High Surface Tension Polar.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Presentation ID1988893 High Surface Tension Polar surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. . High Surface Tension Polar.
From www.learnatnoon.com
What is Surface Tension in Physics? High Surface Tension Polar the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the energy required to increase the. High Surface Tension Polar.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension, heat of vaporization,. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Presentation ID3088888 High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension of water. the surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The attraction of molecules at the surface. High Surface Tension Polar.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download ID6877497 High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. The surface tension of water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download ID2019622 High Surface Tension Polar surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. The surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why. The stronger the intermolecular interactions,. High Surface Tension Polar.
From www2.victoriacollege.edu
polar covalent bonds of water High Surface Tension Polar The polarity of water molecules can help explain why. The surface tension of water. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free download ID2992313 High Surface Tension Polar surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. The polarity of water molecules can help explain why. the surface tension of water. High Surface Tension Polar.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog High Surface Tension Polar The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why. The surface tension of water. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension, heat of vaporization, and. High Surface Tension Polar.
From www.vrogue.co
A Variation Of The Surface Tension And Viscosity Of T vrogue.co High Surface Tension Polar It would take a force of 72 dynes to break a surface film of water 1 cm long. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension. High Surface Tension Polar.
From www.slideserve.com
PPT Unique Properties of water PowerPoint Presentation, free download ID2200516 High Surface Tension Polar surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the. High Surface Tension Polar.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with High Surface Tension in Real High Surface Tension Polar surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. It would take a force of 72 dynes to break a surface film of water 1 cm long. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy,. High Surface Tension Polar.
From stock.adobe.com
surface tension wetting surfactant water hydrophilic hydrophobic surface contact angle Stock High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The surface tension of water. the surface tension of water is 72 dynes/cm at 25°c. The polarity of water molecules can help explain why.. High Surface Tension Polar.
From byjus.com
Explain the surface tension phenomenon with examples. High Surface Tension Polar surface tension is the energy required to increase the surface area of a liquid by a given amount. The attraction of molecules at the surface of a liquid is called surface tension. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a. High Surface Tension Polar.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube High Surface Tension Polar The attraction of molecules at the surface of a liquid is called surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. surface tension. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Liquids and Solids PowerPoint High Surface Tension Polar The stronger the intermolecular interactions, the greater the surface tension. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The polarity of water molecules can help explain why. surface tension is the energy required to increase the surface area of a liquid by. High Surface Tension Polar.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs High Surface Tension Polar The attraction of molecules at the surface of a liquid is called surface tension. The stronger the intermolecular interactions, the greater the surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar. High Surface Tension Polar.
From slideplayer.com
The Respiratory System ppt download High Surface Tension Polar the surface tension of water is 72 dynes/cm at 25°c. The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why. The surface tension of water. surface tension is the energy required to increase the surface. High Surface Tension Polar.
From www.biolinscientific.com
Surface tension of water Why is it so high? High Surface Tension Polar It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the greater the surface tension. Surfactants are molecules, such as soaps. High Surface Tension Polar.
From slideplayer.com
Intermolecular forces ppt download High Surface Tension Polar Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a given amount. The polarity. High Surface Tension Polar.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co High Surface Tension Polar surface tension is the energy required to increase the surface area of a liquid by a given amount. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due. High Surface Tension Polar.
From www.youtube.com
Surface Tension of Water Explained YouTube High Surface Tension Polar surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water. High Surface Tension Polar.
From www.youtube.com
Surface Tension YouTube High Surface Tension Polar surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension. High Surface Tension Polar.
From slideplayer.com
Liquids and Solids “CONDENSED STATES OF MATTER”. ppt download High Surface Tension Polar The attraction of molecules at the surface of a liquid is called surface tension. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the. High Surface Tension Polar.
From slideplayer.com
WATER And Solution Formation ppt download High Surface Tension Polar surface tension is the energy required to increase the surface area of a liquid by a given amount. It would take a force of 72 dynes to break a surface film of water 1 cm long. the surface tension of water is 72 dynes/cm at 25°c. The polarity of water molecules can help explain why. The surface tension. High Surface Tension Polar.
From www.slideserve.com
PPT Measuring Surface Tension PowerPoint Presentation, free download ID6111349 High Surface Tension Polar surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why. The surface tension of water. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension is the force that. High Surface Tension Polar.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free download ID3553753 High Surface Tension Polar Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. surface tension, heat of vaporization, and vapor pressure besides mercury, water has the highest surface tension for all liquids. The surface tension of water. It would take a force of 72 dynes to break a surface film of water 1 cm. High Surface Tension Polar.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American Chemical Society High Surface Tension Polar the surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the force that allows a liquid to resist external forces, and it's high in polar substances like water due to the. The surface tension of water. Surfactants. High Surface Tension Polar.