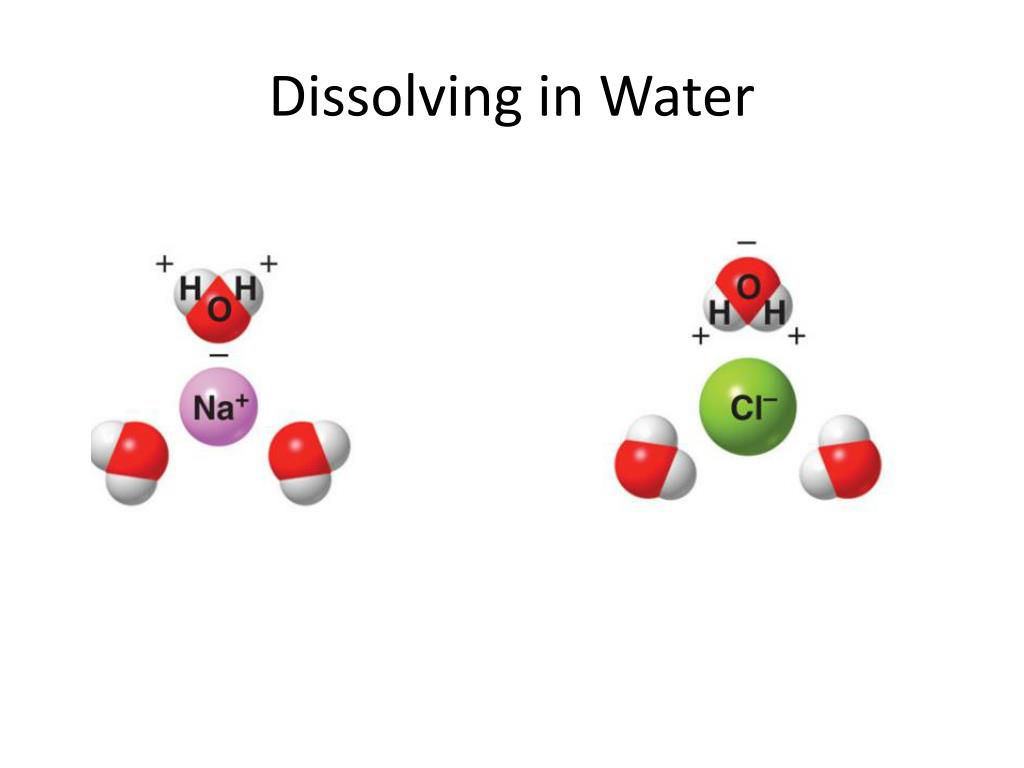
Why Are Ionic Crystals Soluble In Water . Two forces determine the extent to which the solution will occur: ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. (1) the energy change, δhdissolve, that occurs when the ionic. Force of attraction between h2o molecules and the ions of the solid. This force tends to bring ions into solution. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. the solubility of ionic solids in water depends on two things: A considerable high number of ionic compounds are soluble in water. the solubility of ionic solids in water depends of two things: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. The extent to which a substance. solubility in water. (1) the energy change, dedissolve, that occurs when the. predict solubilities of ionic compounds in water using the solubility rules.
from www.slideserve.com
(1) the energy change, dedissolve, that occurs when the. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. the solubility of ionic solids in water depends on two things: Two forces determine the extent to which the solution will occur: solubility in water. (1) the energy change, δhdissolve, that occurs when the ionic. The extent to which a substance. the solubility of ionic solids in water depends of two things: A considerable high number of ionic compounds are soluble in water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic.
PPT Ionic and Covalent bonding PowerPoint Presentation, free download ID3076492
Why Are Ionic Crystals Soluble In Water ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. The extent to which a substance. Force of attraction between h2o molecules and the ions of the solid. predict solubilities of ionic compounds in water using the solubility rules. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. (1) the energy change, dedissolve, that occurs when the. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. A considerable high number of ionic compounds are soluble in water. This force tends to bring ions into solution. Two forces determine the extent to which the solution will occur: the solubility of ionic solids in water depends on two things: solubility in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. (1) the energy change, δhdissolve, that occurs when the ionic. the solubility of ionic solids in water depends of two things:
From wisc.pb.unizin.org
Appendix D Solubility Flow Chart UWMadison Chemistry 103/104 Resource Book Why Are Ionic Crystals Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds in water using the solubility rules. Force of attraction. Why Are Ionic Crystals Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Are Ionic Crystals Soluble In Water ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. the solubility of ionic solids in water depends on two things: A. Why Are Ionic Crystals Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 Why Are Ionic Crystals Soluble In Water The extent to which a substance. the solubility of ionic solids in water depends on two things: predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility. Why Are Ionic Crystals Soluble In Water.
From www.bharatagritech.com
Ions In Aqueous Solution Infographic Diagram Showing, 54 OFF Why Are Ionic Crystals Soluble In Water (1) the energy change, dedissolve, that occurs when the. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. solubility in water. the solubility of ionic solids in water depends of two things: Two forces determine the extent to which the. Why Are Ionic Crystals Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Are Ionic Crystals Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. (1) the energy change, δhdissolve, that occurs when the ionic. A considerable high number of ionic compounds are soluble in water. The extent to which a substance. Force of attraction between h2o molecules and the ions of the solid. predict solubilities. Why Are Ionic Crystals Soluble In Water.
From biology.reachingfordreams.com
Solubility Equilibria and the Solubility Product Constant Biology Why Are Ionic Crystals Soluble In Water (1) the energy change, δhdissolve, that occurs when the ionic. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. A considerable high number of ionic compounds are soluble in water. solubility in water. This force tends to bring ions into solution.. Why Are Ionic Crystals Soluble In Water.
From www.numerade.com
SOLVED Predict whether each of the following ionic compounds is soluble in water Drag the Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends of two things: (1) the energy change, dedissolve, that occurs when the. The extent to which a substance. solubility in water. This force tends to bring ions into solution. the solubility of ionic solids in water depends on two things: when ionic compounds dissolve in water, the ions. Why Are Ionic Crystals Soluble In Water.
From app.jove.com
Solubility Properties of Ionic Compounds Concept Chemistry JoVe Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends on two things: Force of attraction between h2o molecules and the ions of the solid. The extent to which a substance. the solubility of ionic solids in water depends of two things: (1) the energy change, dedissolve, that occurs when the. This force tends to bring ions into solution. . Why Are Ionic Crystals Soluble In Water.
From socratic.org
Substance A will not dissolve in water. What can be said about substance A? Socratic Why Are Ionic Crystals Soluble In Water The extent to which a substance. A considerable high number of ionic compounds are soluble in water. the solubility of ionic solids in water depends of two things: the solubility of ionic solids in water depends on two things: predict solubilities of ionic compounds in water using the solubility rules. solubility in water. when ionic. Why Are Ionic Crystals Soluble In Water.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility chart given in the lab Why Are Ionic Crystals Soluble In Water ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. (1) the energy change, dedissolve, that occurs when the. (1) the energy change, δhdissolve, that. Why Are Ionic Crystals Soluble In Water.
From www.blendspace.com
Chapter 14 Aqueous Solutions Lessons Blendspace Why Are Ionic Crystals Soluble In Water predict solubilities of ionic compounds in water using the solubility rules. solubility in water. Force of attraction between h2o molecules and the ions of the solid. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Two forces determine the extent to which the solution will occur: . Why Are Ionic Crystals Soluble In Water.
From byjus.com
Madam Curie was studying solution chemistry. She was reading that dissolution happens with ionic Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends of two things: Force of attraction between h2o molecules and the ions of the solid. solubility in water. A considerable high number of ionic compounds are soluble in water. The extent to which a substance. the solubility of ionic solids in water depends on two things: solubility is. Why Are Ionic Crystals Soluble In Water.
From www.w3schools.blog
Ionic Solids W3schools Why Are Ionic Crystals Soluble In Water ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. Two forces determine the extent to which the solution will occur: (1) the energy change, δhdissolve, that occurs when the ionic. when ionic compounds dissolve in water, the ions in the solid. Why Are Ionic Crystals Soluble In Water.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure & Properties翰林国际教育 Why Are Ionic Crystals Soluble In Water This force tends to bring ions into solution. the solubility of ionic solids in water depends on two things: solubility in water. the solubility of ionic solids in water depends of two things: predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance. A considerable high number of ionic. Why Are Ionic Crystals Soluble In Water.
From www.chegg.com
Solved 3. Why are some ionic compounds soluble in water and Why Are Ionic Crystals Soluble In Water The extent to which a substance. Force of attraction between h2o molecules and the ions of the solid. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility of ionic solids in water depends on two things: (1) the energy. Why Are Ionic Crystals Soluble In Water.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free download ID1820946 Why Are Ionic Crystals Soluble In Water Force of attraction between h2o molecules and the ions of the solid. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility of ionic solids in water depends on two things: This force tends to bring ions into solution. . Why Are Ionic Crystals Soluble In Water.
From www.thoughtco.com
Solubility Rules of Ionic Solids in Water Why Are Ionic Crystals Soluble In Water The extent to which a substance. (1) the energy change, δhdissolve, that occurs when the ionic. Force of attraction between h2o molecules and the ions of the solid. solubility in water. the solubility of ionic solids in water depends on two things: Two forces determine the extent to which the solution will occur: (1) the energy change, dedissolve,. Why Are Ionic Crystals Soluble In Water.
From saeqvu.blogspot.com
Would The Resulting Solution Conduct Electricity SAEQVU Why Are Ionic Crystals Soluble In Water solubility in water. (1) the energy change, δhdissolve, that occurs when the ionic. Two forces determine the extent to which the solution will occur: (1) the energy change, dedissolve, that occurs when the. This force tends to bring ions into solution. the solubility of ionic solids in water depends on two things: when ionic compounds dissolve in. Why Are Ionic Crystals Soluble In Water.
From bceweb.org
Solubility Of Compounds In Water Chart A Visual Reference of Charts Chart Master Why Are Ionic Crystals Soluble In Water (1) the energy change, dedissolve, that occurs when the. the solubility of ionic solids in water depends of two things: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. This force tends to bring ions into solution. Two forces determine the extent to which the solution will occur: solubility. Why Are Ionic Crystals Soluble In Water.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download ID3076492 Why Are Ionic Crystals Soluble In Water The extent to which a substance. (1) the energy change, dedissolve, that occurs when the. Two forces determine the extent to which the solution will occur: predict solubilities of ionic compounds in water using the solubility rules. the solubility of ionic solids in water depends of two things: Force of attraction between h2o molecules and the ions of. Why Are Ionic Crystals Soluble In Water.
From apchemsite.wordpress.com
Net Ionic Equation Chemistry Geeks Why Are Ionic Crystals Soluble In Water This force tends to bring ions into solution. The extent to which a substance. predict solubilities of ionic compounds in water using the solubility rules. A considerable high number of ionic compounds are soluble in water. solubility in water. the solubility of ionic solids in water depends on two things: Two forces determine the extent to which. Why Are Ionic Crystals Soluble In Water.
From socratic.org
Why are metallic compounds insoluble in water? Socratic Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends of two things: A considerable high number of ionic compounds are soluble in water. (1) the energy change, δhdissolve, that occurs when the ionic. solubility in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. The extent to which a. Why Are Ionic Crystals Soluble In Water.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Compounds AII sodium (Na Why Are Ionic Crystals Soluble In Water (1) the energy change, dedissolve, that occurs when the. A considerable high number of ionic compounds are soluble in water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. solubility in water. Force of attraction between h2o molecules and the ions. Why Are Ionic Crystals Soluble In Water.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends of two things: Force of attraction between h2o molecules and the ions of the solid. predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. (1) the energy change, δhdissolve, that. Why Are Ionic Crystals Soluble In Water.
From kunduz.com
[ANSWERED] Which of the following ionic compounds is soluble in water? Kunduz Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends on two things: solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. The extent to which a substance. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy. Why Are Ionic Crystals Soluble In Water.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Why Are Ionic Crystals Soluble In Water solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. (1) the energy change, δhdissolve, that occurs when the ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. Two forces determine the extent to which the solution will occur: . Why Are Ionic Crystals Soluble In Water.
From www.science-revision.co.uk
Properties of ionic compounds Why Are Ionic Crystals Soluble In Water (1) the energy change, δhdissolve, that occurs when the ionic. A considerable high number of ionic compounds are soluble in water. the solubility of ionic solids in water depends on two things: The extent to which a substance. solubility in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. Why Are Ionic Crystals Soluble In Water.
From chem.libretexts.org
13.2 Solubility and Molecular Structure Chemistry LibreTexts Why Are Ionic Crystals Soluble In Water The extent to which a substance. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Two forces determine the extent to which the solution will occur: the solubility of ionic solids in water depends on two things: This force tends to bring ions into solution. Force of attraction. Why Are Ionic Crystals Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID7002512 Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends on two things: Two forces determine the extent to which the solution will occur: the solubility of ionic solids in water depends of two things: predict solubilities of ionic compounds in water using the solubility rules. (1) the energy change, dedissolve, that occurs when the. when ionic compounds. Why Are Ionic Crystals Soluble In Water.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Why Are Ionic Crystals Soluble In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. A considerable high number of ionic compounds are soluble in water. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Force of attraction between h2o molecules and the ions of the. Why Are Ionic Crystals Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Crystals Soluble In Water the solubility of ionic solids in water depends of two things: ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. The extent to which a substance. the solubility of ionic solids in water depends on two things: A considerable high. Why Are Ionic Crystals Soluble In Water.
From www.chemicals.co.uk
What is Solubility? The Chemistry Blog Why Are Ionic Crystals Soluble In Water The extent to which a substance. predict solubilities of ionic compounds in water using the solubility rules. (1) the energy change, dedissolve, that occurs when the. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. Force of attraction between h2o molecules and the ions of the solid. (1) the energy. Why Are Ionic Crystals Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Crystals Soluble In Water Force of attraction between h2o molecules and the ions of the solid. (1) the energy change, δhdissolve, that occurs when the ionic. This force tends to bring ions into solution. solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. solubility in water. The extent to which a substance.. Why Are Ionic Crystals Soluble In Water.
From www.yourdictionary.com
Ionic Crystal Examples YourDictionary Why Are Ionic Crystals Soluble In Water Force of attraction between h2o molecules and the ions of the solid. the solubility of ionic solids in water depends on two things: solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. (1) the energy change, dedissolve, that occurs when the. The extent to which a substance. (1). Why Are Ionic Crystals Soluble In Water.
From exogqaefj.blob.core.windows.net
Copper Chloride Water Solubility at Sandra Barrera blog Why Are Ionic Crystals Soluble In Water solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. predict solubilities of ionic compounds in water using the solubility rules. Force of attraction between h2o molecules and the ions of the solid. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Why Are Ionic Crystals Soluble In Water.